Fluphenazine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
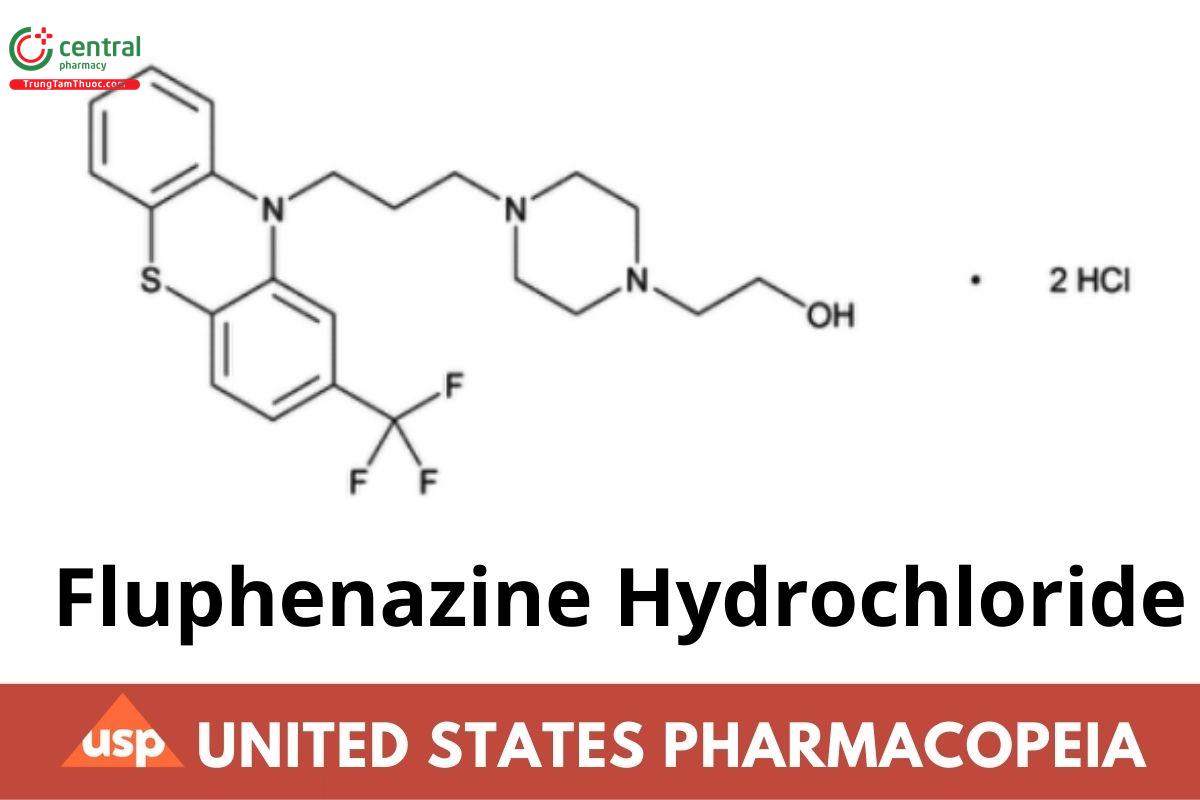
C22H26F3N3OS · 2HCl 510.44
1-Piperazineethanol, 4-[3-[2-(trifluoromethyl)-10H- phenothiazin-10-yl]propyl]-, dihydrochloride;
4-[3-[2-(Triuoromethyl)phenothiazin-10-yl]propyl]-1- piperazineethanol dihydrochloride CAS RN®: 146-56-5; UNII: ZOU145W1XL.
1 DEFINITION
Fluphenazine Hydrochloride contains NLT 97.0% and NMT 103.0% of fluphenazine hydrochloride (C22H26F3N3OS · 2HCl), calculated on the dried basis.
[Note—Throughout the following procedures, protect samples, the Reference Standard, and solutions containing them by conducting the procedures without delay, under subdued light, or using low-actinic glassware.]
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
Change to read:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Analytical wavelength: 259 nm
Sample solution: 10 µg/mL of Fluphenazine Hydrochloride in methanol
Acceptance criteria: Absorptivities, calculated on the dried basis, do not differ by more than 2.5%.
C. Identification Tests—General, Chloride〈191〉: Meets the requirements
3 ASSAY
Procedure
Buffer: 0.05 M monobasic potassium phosphate adjusted with phosphoric acid to a pH of 2.5
Diluent: Acetonitrile, methanol, and Buffer (30:30:40)
Mobile phase: 0.2% triethylamine in Diluent
Standard solution: 0.06 mg/mL of USP Fluphenazine Hydrochloride RS in Diluent
Sample stock solution: 1.2 mg/mL of Fluphenazine Hydrochloride in Diluent
Sample solution: 0.06 mg/mL of Fluphenazine Hydrochloride from Sample stock solution in Diluent. Filter, and discard the first 5 mL of the filtrate.
3.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4-mm × 12.5-cm; packing L7
Flow rate: 1 mL/min
Injection volume: 25 µL
3.2 System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 2000 theoretical plates
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
3.3 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of fluphenazine hydrochloride (C22H26F3N3OS · 2HCl) in the portion of Fluphenazine Hydrochloride taken:
Result = (rU /rS) × (CS /CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Fluphenazine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Fluphenazine Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.5%
Ordinary Impurities 〈466〉
Standard solution and Test solution: 0.1 M methanolic sodium hydroxide
Eluant: Acetone, cyclohexane, and diethylamine (40:15:1)
Visualization: 1
Acceptance criteria: Meets the requirements
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry at 65° for 3 h.
Acceptance criteria: NMT 1%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
USP Reference Standards 〈11〉
USP Fluphenazine Hydrochloride RS.


