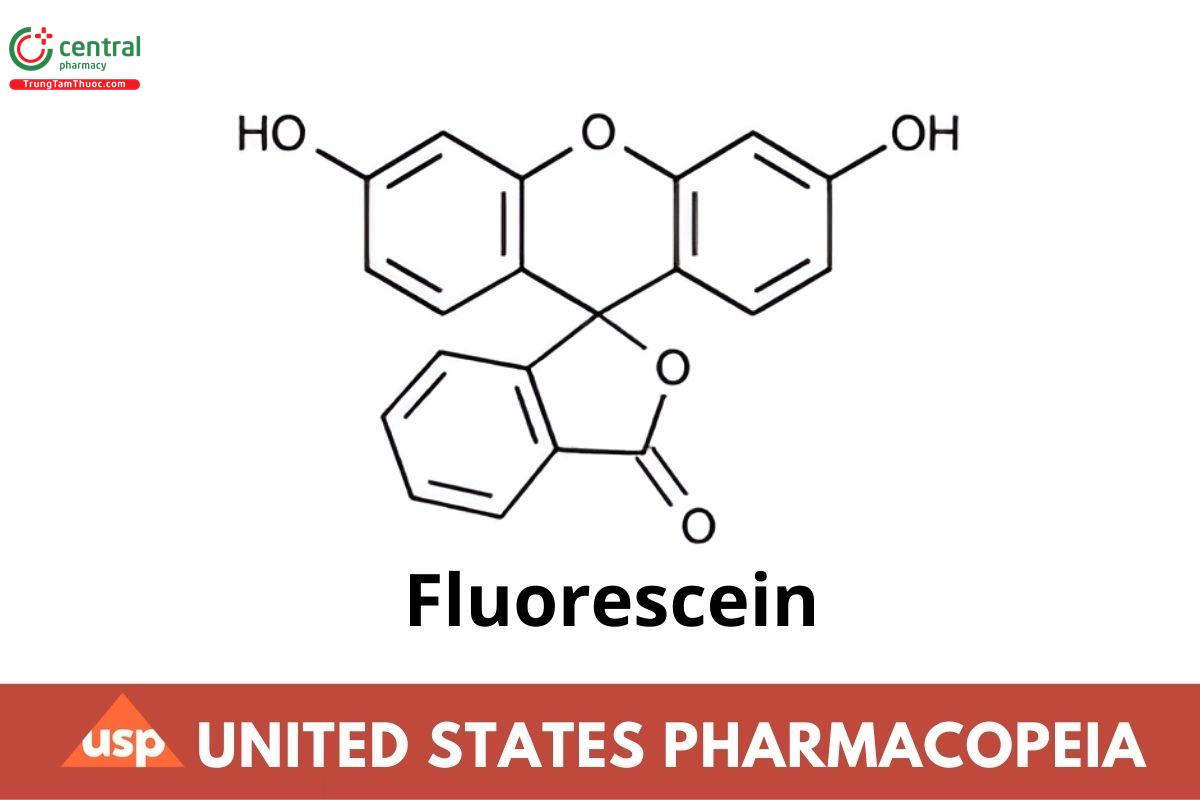
Fluorescein
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C20H12O5 332.31
Spiro[isobenzofuran-1(3H),9′-[9H]xanthen-3-one, 3′,6′-dihydroxy-.
Fluorescein CAS RN®: 2321-07-5.
Fluorescein contains not less than 97.0 percent and not more than 102.0 percent of C20H12O5 calculated on the anhydrous basis.
1 Packaging and storage
Preserve in tight containers.
USP Reference standards 〈11〉
USP Diacetylfluorescein RS C24H16O7 416.39
USP Fluorescein RS
Change to read:
2 Identification
Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020) : previously dried over silica gel for 16 hours.
Water Determination, Method I 〈921〉: not more than 1.0%.
3 Zinc
Suspend 100 mg in 10 mL of a saturated solution of sodium chloride, add 2 mL of 3 N hydrochloric acid, mix, filter, and add 1 mL of potassium ferrocyanide TS to the filtrate: no turbidity is produced.
4 Acrifiavine
Suspend 10 mg in 5 mL of water, swirl the mixture, and filter. To the filtrate add a few drops of sodium salicylate solution (1 in 10): no precipitate is formed.
5 Assay
Standard preparation-Dissolve about 110 mg of USP Diacetylfluorescein RS, accurately weighed, in 10 mL of alcohol contained in a 100-mL volumetric flask. Add 2 mL of 2.5 N sodium hydroxide, and heat on a steam bath at about the boiling temperature for 20 minutes, with frequent swirling. Cool, dilute with water to volume, and mix. Dilute quantitatively and stepwise with water to obtain a solution having a known concentration of about 1.1 μg of diacetyl fluorescein per mL. Transfer 3.0 mL of this solution to a 100-mL volumetric flask containing 20 mL of pH 9.0 alkaline borate buffer (see Buffer Solutions in the section Reagents, Indicators, and Solutions), dilute with water to volume, and mix.
Assay preparation - Dissolve about 90 mg of Fluorescein, accurately weighed, in 10 mL of alcohol contained in a 100-mL volumetric flask. Add 2 mL of 2.5 N sodium hydroxide, and heat on a steam bath at about the boiling temperature for 20 minutes, with frequent swirling. Cool, dilute with water to volume, and mix. Dilute quantitatively and stepwise with water to obtain a solution having a concentration of 0.9 μg per mL. Transfer 3.0 mL of this solution to a 100-mL volumetric flask containing 20 mL of pH 9.0 alkaline borate buffer (see Buffer Solutions in the section Reagents, Indicators, and Solutions), dilute with water to volume, and mix.
6 Procedure
Concomitantly determine the fluorescence intensities, I, of the Standard preparation and the Assay preparation in a fluorometer at an excitation wavelength of 485 nm and an emission wavelength of 515 nm. Calculate the quantity, in mg, of C H O in the Fluorescein taken by the formula:
(332.31/416.39)(3333C)(IU/IS)
in which 332.31 and 416.39 are the molecular weights offluorescein and diacetyl fluorescein, respectively; C is the concentration, in μg per mL, of USP Diacetyl fluorescein RS in the Standard preparation; and IU and IS are the fluorescence values observed for the Assay preparation and the Standard preparation, respectively.


