Flumazenil
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
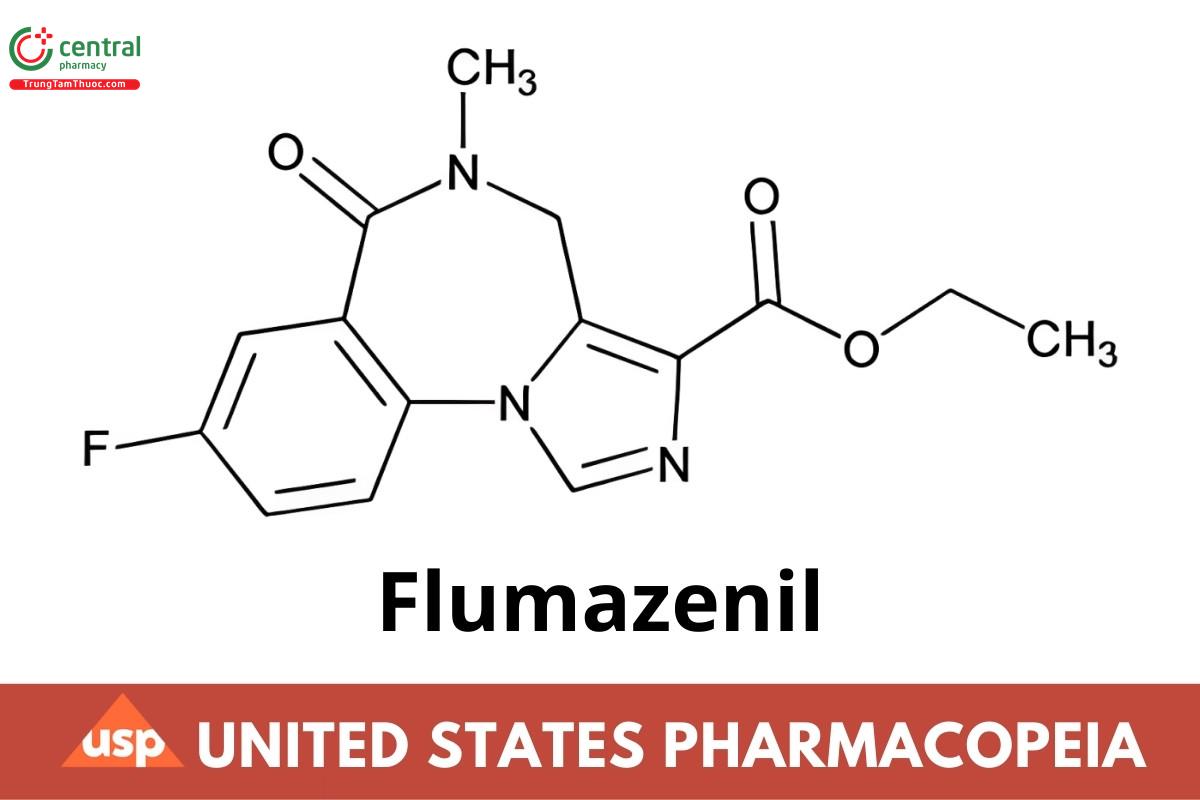
C15H14FN3O3 303.29
4H-Imidazo[1,5-a][1,4] benzodiazepine-3-carboxylic acid, 8-fluoro-5,6-dihydro-5-methyl-6-oxo-, ethyl ester;
Ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate
CAS RN®: 78755-81-4; UNII: 40P7XK9392.
1 DEFINITION
Flumazenil contains NLT 98.0% and NMT 102.0% of flumazenil (C15H14FN3O3), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Solution A: 800 mL of water, adjusted with phosphoric acid to a pH of 2.0 ± 0.05
Mobile phase: Methanol, tetrahydrofuran, and Solution A (13:7:80)
System suitability solution: 6.4 µg/mL each of USP Flumazenil RS and USP Flumazenil Related Compound B RS in Mobile phase
Standard solution: 1.0 mg/mL of USP Flumazenil RS in Mobile phase
Sample solution: 1.0 mg/mL of Flumazenil in Mobile phase
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 5 µL
Run time: NLT 1.3 times the
3.3 System suitability
Samples: System suitability solution and Standard solution
[NOTE-See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 4.0 between flumazenil related compound B and flumazenil, System suitability solution
Tailing factor: NMT 1.5 for flumazenil, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
3.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of flumazenil (C15H14FN3O3) in the portion of Flumazenil taken:
Result = (rU/rS) x (CS/CU) x 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Flumazenil RS in the Standard solution (mg/mL)
CU = concentration of Flumazenil in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%
Change to read:
4.2 LIMIT OF FLUMAZENIL RELATED COMPOUND C
Diluent: Alcohol and chloroform (50:50)
Standard solution A: 0.5 mg/mL of USP Flumazenil RS and 0.6 µL/mL of USP Flumazenil Related Compound C RS in Diluent
Standard solution B: 0.1 mg/mL of USP Flumazenil RS and 0.12 µL/mL (ERR 1-Jun-2018) of USP Flumazenil Related Compound C RS from Standard solution A in Diluent
Sample solution: 50 mg/mL of Flumazenil in Diluent
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL
Developing solvent system: Chloroform, glacial acetic acid, alcohol, and water (75:15:7.5:2.5)
Spray reagent: Dissolve 0.5 g of ninhydrin in 90 mL of alcohol, and add 10 mL of glacial acetic acid.
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Proceed as directed in the chapter. Dry the plate for 10 min in a current of cold air, and view under short-wavelength UV light. Spray the plate with Spray reagent, and heat at 105° for 15 min. The RF values of the analytes with their corresponding methods of detection are listed in Table 1.
Table 1
| Compound | RF | Detection |
| Flumazenil | About 0.8 | UV |
| Flumazenil related compound C | About 0.04 | Ninhydrin |
Acceptance criteria: Any spot at an R, value corresponding to flumazenil related compound C of the Sample solution is not more intense than the corresponding spot of Standard solution B (NMT 0.2%).
4.3 ORGANIC IMPURITIES
Solution A, Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay except use a Run time of NLT 3 times the retention time of flumazenil.
Standard solution: 1 µg/mL of USP Flumazenil RS in Mobile phase
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of each impurity in the portion of Flumazenil taken:
Result = (rU/rS) x (CS/CU) x (1/F) x 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Flumazenil RS in the Standard solution (mg/mL)
CU = concentration of Flumazenil in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Flumazenil related compound Aa | 0.4 | 1.1 | 0.2 |
| Flumazenil dioneb | 0.5 | 1.5 | 0.2 |
| Desuoro flumazenilc | 0.7 | 1.3 | 0.2 |
| Flumazenil related compound B | 0.8 | 1.1 | 0.2 |
| Flumazenil | 1.0 | — | — |
| Chlorouflumazenild | 2.2 | 1.1 | 0.2 |
| Any individual unknown impurity | — | 1.0 | 0.1 |
| Total impurities | — | — | 0.5 |
a 8-Fluoro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid; also known as 8-Fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazol-[1,5-a][1,4]benzodiazepine-3-carboxylic acid.
b 7-Fluoro-4-methyl-3,4-dihydro-1H-benzo[e][1,4]diazepine-2,5-dione.
c Ethyl 5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate.
d Ethyl 8-chloro-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate.
5 SPECIFIC TESTS
5.1 BACTERIAL ENDOTOXINS TEST (85)
NMT 25.0 USP Endotoxin Units/mg of flumazenil
5.2 LOSS ON DRYING (731)
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in tight containers, and store at controlled room temperature.
6.2 USP REFERENCE STANDARDS (11)
USP Flumazenil RS
USP Flumazenil Related Compound B RS
Ethyl 8-hydroxy-5-methyl-6-oxo-5,6-dihydro-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate; also known as Ethyl 8-hydroxy-5,6-dihydro-5-methyl-6-oxo-4H-imidazol-[1,5-a] [1,4]benzodiazepine-3-carboxylate.
C15H15N3O4 301.30
USP Flumazenill Related Compound C RS
1,1-Diethyoxy-N,N-dimethylmethanamine;
also known as N,N-Dimethylformamide diethyl acetal.
C7H17NO2 147.22


