Floxuridine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
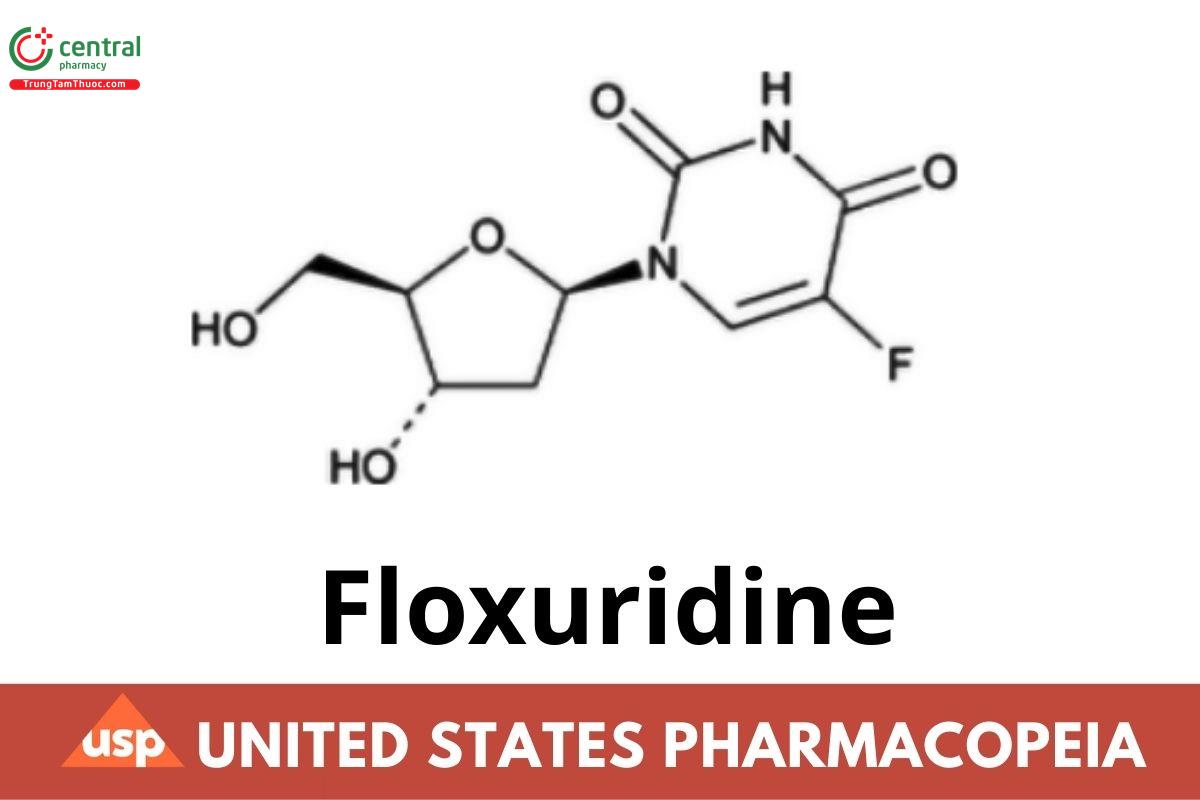
C9H11FN2O5 246.19
Uridine, 2'-deoxy-5-uoro-;
2'-Deoxy-5-uorouridine CAS RN®: 50-91-9; UNII: 039LU44I5M.
1 DEFINITION
Floxuridine contains NLT 98.5% and NMT 101.0% of floxuridine (C9H11FN2O5), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M (CN 1-May-2020)
Change to read:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Analytical wavelength: 268 nm
Sample solution: 20 µg/mL
Medium: 0.1 N potassium hydroxide
Acceptance criteria: Absorptivities, calculated on the dried basis, do not differ by more than 3.0%.
C.
Sample solution: 20 mg/mL of Floxuridine in water
Analysis: Add a few drops of bromine TS to 10 mL of the Sample solution.
Acceptance criteria: The bromine color is discharged.
3 ASSAY
Procedure
Sample: 800 mg of Floxuridine
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N tetrabutylammonium hydroxide VS
Endpoint detection: Potentiometric
Analysis: Dissolve the Sample in 80 mL of dimethylformamide, and titrate with Titrant, determining the endpoint using a calomel–glass electrode system. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N tetrabutylammonium hydroxide is equivalent to 24.62 mg of floxuridine (C9H11FN2O5).
Acceptance criteria: 98.5%–101.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Limit of Fluoride Ions
Use plasticware throughout this test.
Buffer: Dissolve 110 g of sodium chloride and 1 g of sodium citrate in 700 mL of water in a 2000-mL volumetric ask. Cautiously add 150 g of sodium hydroxide, and dissolve with shaking. Cool to room temperature, and while stirring, cautiously add 450 mL of glacial acetic acid to the cooled solution. Cool, add 600 mL of isopropyl alcohol, and dilute with water to volume. The pH of the Buffer is 5.0–5.5.
Standard stock solution: Transfer 221 mg of sodium fluoride, previously dried at 150° for 4 h, to a 100-mL volumetric ask, add 20 mL of water, and mix to dissolve. Add 1.0 mL of sodium hydroxide solution (1 in 2500), dilute with water to volume, and mix. Each mL of the Standard stock solution contains 1 mg of fluoride ions. Store in a tightly closed, plastic container.
Standard solutions: 1, 3, 5, and 10 µg/mL of fluoride ion; from Standard stock solution in Buffer
Sample solution: 10 mg/mL of Floxuridine in Buffer
Analysis: Concomitantly measure the potential (see Titrimetry 〈541〉), in mV, of the Standard solutions and the Sample solution, with a pH meter capable of a minimum reproducibility of ±0.2 mV, equipped with a glass-sleeved calomel–uoride specic-ion electrode system. When taking measurements, immerse the electrodes in the solution, which has been transferred to a 150-mL beaker containing a polytef coated stirring bar. Allow to stir on a magnetic stirrer having an insulated top until equilibrium is attained (1–2 min), and record the potential. Rinse and dry the electrodes between measurements, taking care to avoid damaging the crystal of the specic-ion electrode. Plot the logarithm of the uoride-ion concentrations, in µg/mL, of the Standard solutions versus potential, in mV. From the measured
potential of the Sample solution and the standard curve, determine the concentration, in µg/mL, of fluoride ions in the Sample solution. Acceptance criteria: NMT 0.05%
5 SPECIFIC TESTS
Melting Range or Temperature, Class I〈741〉: 145°–153°, but the range between the beginning and end of melting does not exceed 2°.
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL of Floxuridine in water.
Acceptance criteria: +36° to +39°
Loss on Drying 〈731〉
Analysis: Dry under vacuum over silica gel at 60° for 4 h.
Acceptance criteria: NMT 0.2%
Pyrogen: Where the label states that Floxuridine is sterile, it meets the requirements of Pyrogen Test 〈151〉, the test dose being 0.50 mL/kg of a solution prepared by diluting Floxuridine with Sodium Chloride Injection to a concentration of 100 mg/mL.
Other Requirements: Where the label states that Floxuridine is sterile, it meets the requirements in Packaging and Storage Requirements 〈659〉, Injection Packaging, Packaging for constitution. Where the label states that Floxuridine must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for the Pyrogen test.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store at 25°, excursions permitted between 15° and 30°.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected for further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Floxuridine RS.


