Famotidine
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
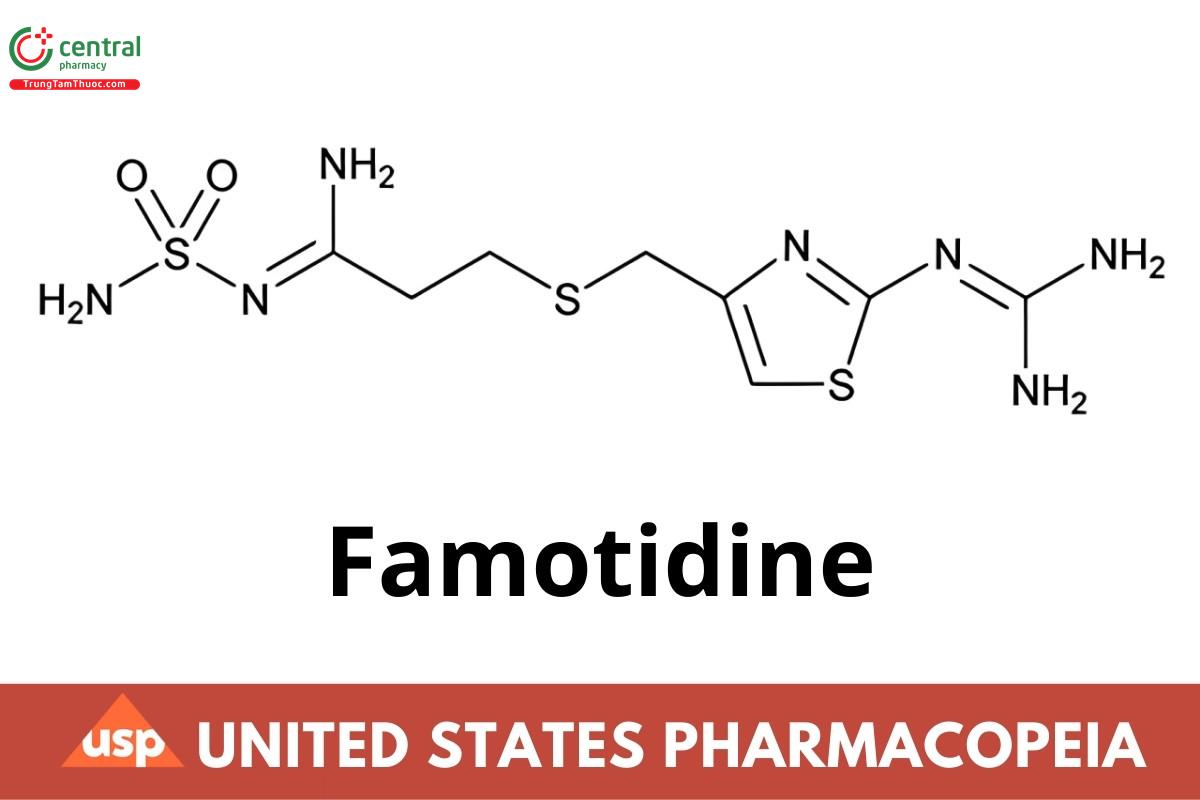
C8H15N7O2S3 337.45
Propanimidamide, N'-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]-;
[1-Amino-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propylidene]sulfamide;
3-[[2-(Diaminomethyleneamino)thiazol-4-yl]methylthio]-N'-sulfamoylpropanimidamide CAS RN®: 76824-35-6; UNII: 5QZO15J2Z8.
1 DEFINITION
Famotidine contains NLT 98.5% and NMT 101.0% of famotidine (C8H15N7O2S3), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy, 1974, 197K, or 197M (CN 1-May-2020)
3 ASSAY
PROCEDURE
Sample: Dissolve 250 mg of Famotidine in 80 mL of glacial acetic acid.
Analysis: Titrate with 0.1 N perchloric acid VS (see Titrimetry (541)), using a suitable anhydrous electrode system. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 16.87 mg of C8H15N7O2S3.
Acceptance criteria: 98.5%-101.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%
4.2 ORGANIC IMPURITIES
Buffer: 1.882 g/L of sodium 1-hexanesulfonate in water, adjusted with acetic acid to a pH of 3.5
Solution A: Acetonitrile, methanol, and Buffer (94:6:900)
Solution B: Acetonitrile
Mobile phase: See Table 1. [NOTE-If necessary, adjust the Mobile phase to achieve a retention time of 19-23 min for the famotidine peak and a maximum of 48 min for the famotidine related compound E peak.]
Table 1
| Time (min) | Solution A (%) | Solution B (%) | Flow Rate (mL/min) |
| 0 | 100 | 0 | 1 |
| 23 | 96 | 4 | 1 |
| 27 | 96 | 4 | 2 |
| 47 | 78 | 22 | 2 |
| 48 | 100 | 0 | 2 |
| 54 | 100 | 0 | 1 |
Standard stock solution: 0.5 mg/mL of USP Famotidine RS in Solution A
Standard solution: 0.5 µg/mL of USP Famotidine RS in Solution A
System suitability stock solution: 0.25 mg/mL of USP Famotidine Related Compound D RS in methanol
System suitability solution: Transfer 1 mL of the System suitability stock solution and 0.5 mL of the Standard stock solution into a 100-mL volumetric flask, and dilute with Solution A to volume.
Sample solution: 0.5 mg/mL of Famotidine in Solution A
Identification solution: 0.5 mg/mL of USP Famotidine RS and 1.5 µg/mL of each of USP Famotidine Related Compound B RS, USP Famotidine Related Compound C RS, USP Famotidine Related Compound D RS. USP Famotidine Related Compound E RS, and USP Famotidine Related Compound F RS in Solution A
[NOTE-To address the poor solubility of USP Famotidine Related Compound F RS in Solution A, it may be first dissolved in a minimal amount of 0.1 N sodium hydroxide.]
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 265 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 50°
Flow rate: See Table 1.
Injection volume: 20 µL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 3.5 between famotidine and famotidine related compound D
Analysis
Samples: Standard solution, Sample solution, and Identification solution
Chromatograph the Identification solution, and identify the components on the basis of their relative retention times, given in Table 2. Calculate the percentage of each impurity in the portion of Famotidine taken:
Result = (rU/rS) x (CS/CU) x (1/F) x 100
rU = peak response of each impurity from the Sample solution
rS = peak response of famotidine from the Standard solution
CS = concentration of USP Famotidine RS in the Standard solution (mg/mL)
CU = concentration of Famotidine in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Famotidine | 1.0 | — | — |
| Famotidine related compound Da | 1.1 | 1.0 | 0.3 |
| Famotidine related compound Cb | 1.2 | 0.53 | 0.3 |
| Famotidine cyanoamidinec | 1.4 | 0.71 | 0.2 |
| Famotidine related compound Fd | 1.5 | 0.59 | 0.1 |
| Famotidine amidinee | 1.6 | 0.53 | 0.2 |
| Famotidine related compound Bf | 2.0 | 0.40 | 0.3 |
| Famotidine related compound Eg | 2.1 | 1.0 | 0.3 |
| Any other individual impurity | — | 1.0 | 0.1 |
| Total impurities | — | — | 1.0 |
a Famotidine propanamide.
b Famotidine sulfamoyl propanamide.
c N-Cyano-3-[[2-(diaminomethyleneamino)thiazol-4-yl]methylthio]propanimidamide.
d Famotidine propionic acid.
e 3-[[2-(Diaminomethyleneamino)thiazol-4-yl]methylthio]propanimidamide.
f Famotidine dimer.
g Famotidine disulfide.
5 SPECIFIC TESTS
LOSS ON DRYING (731).
Analysis: Dry a sample at a pressure not exceeding 5 mm of mercury at 80° for 5 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
6.1 PACKAGING AND STORAGE
Preserve in well-closed containers, protected from light. Store at room temperature.
6.2 USP REFERENCE STANDARDS (11)
USP Famotidine RS
USP Famotidine Related Compound B RS
3,5-Bis[2-[[2-[(diaminomethylene)amino]thiazol-4-yl]methylthio]ethyl]-4H-1,2,4,6-thiatriazine 1,1-dioxide.
C16H23N11O2S5 561.73
USP Famotidine Related Compound C RS
3-[[2-(Diaminomethyleneamino)thiazol-4-yl]methylthio]-N-sulfamoylpropanamide hydrochloride.
C8H13CIN6O3S3 374.88
USP Famotidine Related Compound D RS
3-[[2-(Diaminomethyleneamino) thiazol-4-yl]methylthio]propanamide.
C8H13N5OS2 259.35
USP Famotidine Related Compound E RS
2,2'-[4,4'-Disulfanediylbis(methylene)bis(thiazole-4,2-diyl)]diguanidine.
C10H14N8S4 374.53
USP Famotidine Related Compound F. RS
3-[[2-(Diaminomethyleneamino)thiazol-4-yl]methylthio]propanoic acid.
C8H12N4O2S2 260.34


