Everolimus
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
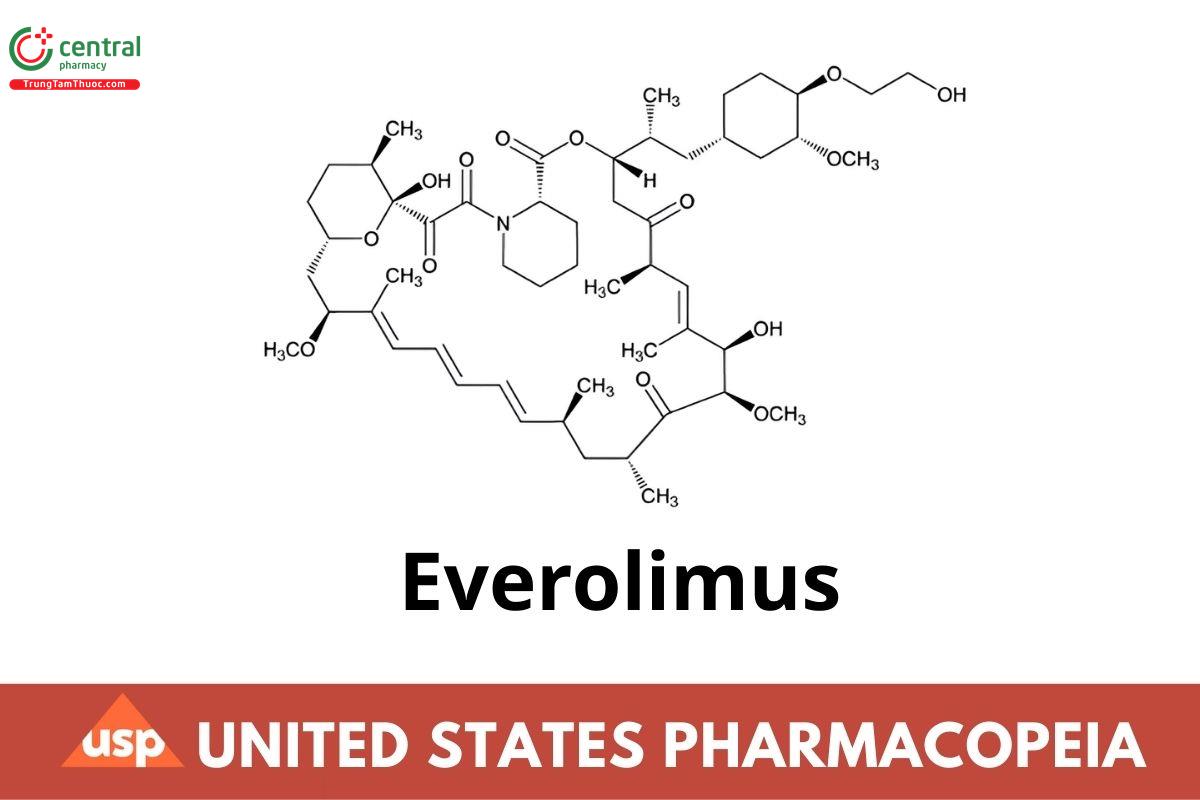
C53H83NO14 958.24 (CN 1-Aug-2024)
(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-Dihydroxy-12-[(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone;
(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone CAS RN®: 159351-69-6.
1 DEFINITION
Everolimus contains NLT 95.0% and NMT 102.0% of everolimus (C53H83NO14), calculated on the anhydrous and solvent-free basis. It may contain a suitable antioxidant.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Protect everolimus from exposure to oxygen. Protect solutions containing everolimus from light.
Solution A: 0.27 g/L of monobasic potassium phosphate
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 60 | 40 |
| 5 | 43 | 57 |
| 23 | 36 | 64 |
| 31 | 0 | 100 |
| 36 | 0 | 100 |
| 37 | 60 | 40 |
| 45 | 60 | 40 |
System suitability solution: 0.5 mg/mL of USP Everolimus System Suitability Mixture RS in acetonitrile
Standard solution: 0.5 mg/mL of USP Everolimus RS in acetonitrile
Sample solution: 0.5 mg/mL of Everolimus in acetonitrile
3.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 275 nm
Column: 3-mm × 25-cm; 5-μm packing L1
Column temperature: 50°
Flow rate: 1.1 mL/min
Injection volume: 10 μL
3.3 System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for 9-O-hydroxyethyl sirolimus, everolimus, and everolimus oxepane isomer are about 0.92, 1.0, and 1.1,respectively.]
3.4 Suitability requirements
Resolution: NLT 1.2 between 9-O-hydroxyethyl sirolimus and everolimus and NLT 1.5 between everolimus and everolimus oxepane isomer,
3.5 System suitability solution
Relative standard deviation: NMT 2.0% for the sum of the everolimus and everolimus oxepane isomer peaks, Standard solution
3.6 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of everolimus (C53H83NO14) in the portion of Everolimus taken:
Result = [(rU /rS ) × (CS /CU ) × 100] − F
rU = sum of the peak responses of everolimus and everolimus oxepane isomer from the Sample solution
rS = sum of the peak responses of everolimus and everolimus oxepane isomer from the Standard solution
CS = concentration of USP Everolimus RS in the Standard solution (mg/mL)
CU = concentration of Everolimus in the Sample solution (mg/mL)
F = percentage of sirolimus from the test for Limit of Sirolimus
Acceptance criteria: 95.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
4.1 Limit of Alcohol
Analysis: See Residual Solvents 〈467〉.
Acceptance criteria: NMT 1.5%
4.1.1 Organic Impurities
Protect everolimus from exposure to oxygen. Protect solutions containing everolimus from light and inject them within 2 h of preparation to minimize the effects of isomerization between everolimus and everolimus oxepane isomer.
Solution A, Solution B, and Mobile phase: Prepare as directed in the Assay.
System suitability solution: 5 mg/mL of USP Everolimus System Suitability Mixture RS in acetonitrile. Use the solution within 24 h.
Sensitivity solution: 0.0025 mg/mL of USP Everolimus RS in acetonitrile
Standard solution: 0.05 mg/mL of USP Everolimus RS in acetonitrile
Sample solution: 5 mg/mL of Everolimus in acetonitrile
4.1.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 3-mm × 25-cm; 5-μm packing L1
Column temperature: 50°
Flow rate: 1.1 mL/min
Injection volume: 10 μL
4.1.3 System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[Note—See Table 2 for relative retention times.]
4.1.4 Suitability requirements
Resolution: NLT 1.2 between 9-O-hydroxyethyl sirolimus and everolimus; NLT 1.5 between everolimus and everolimus oxepane isomer,
4.1.5 System suitability solution
Relative standard deviation: NMT 5% for everolimus, Standard solution
Signal-to-noise ratio: NLT 10 for everolimus, Sensitivity solution
4.1.6 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Everolimus taken:
Result = (rU /rS ) × (CS /CU ) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of everolimus from the Standard solution
CS = concentration of USP Everolimus RS in the Standard solution (mg/mL)
CU = concentration of Everolimus in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. The reporting threshold is 0.05%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Everolimus-19-ene open ringᵃ | 0.4 | 1.25 | 0.5 |
| Specified unidentified impurity 1 | 0.75 | 1.0 | 0.4 |
| Specified unidentified impurity 2 | 0.78 | 1.0 | 0.4 |
| Specified unidentified impurity 3 | 0.81 | 1.0 | 0.8 |
| 10-Desmethyl everolimusᵇ | 0.87 | 1.0 | 0.8 |
| 9-O-Hydroxyethyl sirolimusᶜ | 0.92 | 1.0 | 0.8 |
| Everolimus | 1.0 | – | – |
| Everolimus oxepane isomerᵈ,ᵉ | 1.1 | – | – |
| Butylated hydroxytolueneᶠ | 1.2 | – | – |
| Formyl sirolimusᵍ and specified unidentified impurity 4ʰ | 1.4 | 1.0 | 0.3 |
| Everolimus cyclic hemiacetalⁱ | 1.6 | 1.25 | 0.2 |
| Any individual unspecified impurity | – | 1.0 | 0.20 |
| Total impurities | – | – | 2.5 |
a(S)-1-{2-[(2R,3R,6S)-2-Hydroxy-6-{(2S,3E,5E,7E,9S,11R,13R,14R,15E,17R,19E,21R)-14-hydroxy-22-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-2,13-dimethoxy-3,9,11,15,17,21-hexamethyl-12,18-dioxodocosa-3,5,7,15,19-pentaen-1-yl}-3-methyltetrahydro-2H-pyran-2-yl]-2-oxoacetyl}piperidine-2-carboxylic acid.
b(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,10,27-trihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl]-21-methoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone.
c(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-27-hydroxy-9-(2-hydroxyethoxy)-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]-oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone.
d3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,28R,34aS)-9,10,12,13,14,21,22,23,24,25,26,28,32,33,34,34a-Hexadecahydro-9,28-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,28-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,27,29(4H,6H,31H)-pentone.
e Everolimus oxepane isomer is formed in polar protic solvents. It is listed here for information only; it is not to be reported.
f Butylated hydroxytoluene is an antioxidant that may be used to stabilize everolimus. It is listed here for information only; it is not to be reported.
g(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-formyloxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone.
h The system may resolve formyl sirolimus and specified impurity 4. The limit is for the sum of the two peaks.
i(3S,6R,7E,9R,10R,11R,12R,14S,15S,16E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,15,18,21,22,23,24,25,26,27,32,33,34,34a-Octadecahydro-9,11,27-trihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-18-ethoxy-6,8,12,14,20,26-hexamethyl-23,27:11,15-diepoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,28,29(4H,6H,31H)-tetrone.
4.2 Limit of Sirolimus
Protect everolimus from exposure to oxygen. Protect solutions containing everolimus from light.
Solution A: 0.32 g of ammonium formate dissolved in 450 mL of water, 100 mL of methanol, 450 mL of acetonitrile, and 0.05 mL of formic acid
Solution B: 0.32 g of ammonium formate dissolved in 330 mL of water, 100 mL of methanol, 570 mL of acetonitrile, and 0.05 mL of formic acid
Mobile phase: See Table 3. [Note—The procedure is sensitive to the percentage of methanol in the Mobile phase.]
Table 3
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 14 | 100 | 0 |
| 23 | 0 | 100 |
| 26 | 0 | 100 |
| 26.5 | 100 | 0 |
| 32 | 100 | 0 |
System suitability solution: 2 mg/mL of USP Everolimus System Suitability Mixture RS and 0.016 mg/mL of USP Sirolimus RS in acetonitrile
Sensitivity solution: 0.001 mg/mL of USP Everolimus RS in acetonitrile
Standard solution: 0.016 mg/mL of USP Everolimus RS in acetonitrile
Sample solution: 2 mg/mL of Everolimus in acetonitrile
4.2.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 278 nm
Column: 2.1-mm × 15-cm; 2.7-μm packing L1
4.2.2 Temperatures
[Note—A detector temperature of 40° was shown to improve the chromatography.]
Autosampler: 6°
Column: 60°
Flow rate: 0.9 mL/min
Injection volume: 3.5 μL
4.2.3 System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[Note—See Table 4 for relative retention times.]
4.2.4 Suitability requirements
Resolution: NLT 1.2 between sirolimus and everolimus, System suitability solution
Relative standard deviation: NMT 5% for the sum of the peak responses for everolimus and everolimus oxepane isomer, Standard solution
Signal-to-noise ratio: NLT 10 for the everolimus peak, Sensitivity solution
4.2.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of sirolimus in the portion of Everolimus taken:
Result = (rU /rS ) × (CS /CU ) × 100
rU = peak response of sirolimus from the Sample solution
rS = sum of the peak responses of everolimus and everolimus oxepane isomer from the Standard solution
CS = concentration of USP Everolimus RS in the Standard solution (mg/mL)
CU = concentration of Everolimus in the Sample solution (mg/mL)
Acceptance criteria: See Table 4. The reporting threshold is 0.05%.
Table 4
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Everolimus-19-ene open ringᵃ | 0.4 | – |
| 10-Desmethyl everolimusᵃ | 0.7 | – |
| Sirolimusᵇ | 0.9 | 0.8 |
| Everolimus | 1.0 | – |
| Everolimus oxepane isomerᶜ | 1.1 | – |
a These impurities are controlled in the test for Organic Impurities. They are listed here for information only.
b(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]-oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone.
c Everolimus oxepane isomer is formed in polar protic solvents. It is listed here for information only; it is not to be reported.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 1.5%
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 10 mg/mL in methanol
Acceptance criteria: −141.0° to −153.0° on the anhydrous basis
Microbial Enumeration Tests 〈61〉 and Tests for Specified Organisms 〈62〉: The total aerobic microbial count does not exceed 103cfu/g. The total yeasts and molds count does not exceed 102 cfu/g.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers in an atmosphere of protective gas. Store at refrigerated temperatures.
Labeling: Label it to indicate the nature and the content of the antioxidant, if present.
USP Reference Standards 〈11〉
USP Everolimus RS
USP Everolimus System Suitability Mixture RS
This is a mixture of everolimus, everolimus oxepane isomer, and 9-O-hydroxyethyl sirolimus. Other minor components may be present.
USP Sirolimus RS


