Etidronate Disodium
If you find any inaccurate information, please let us know by providing your feedback here
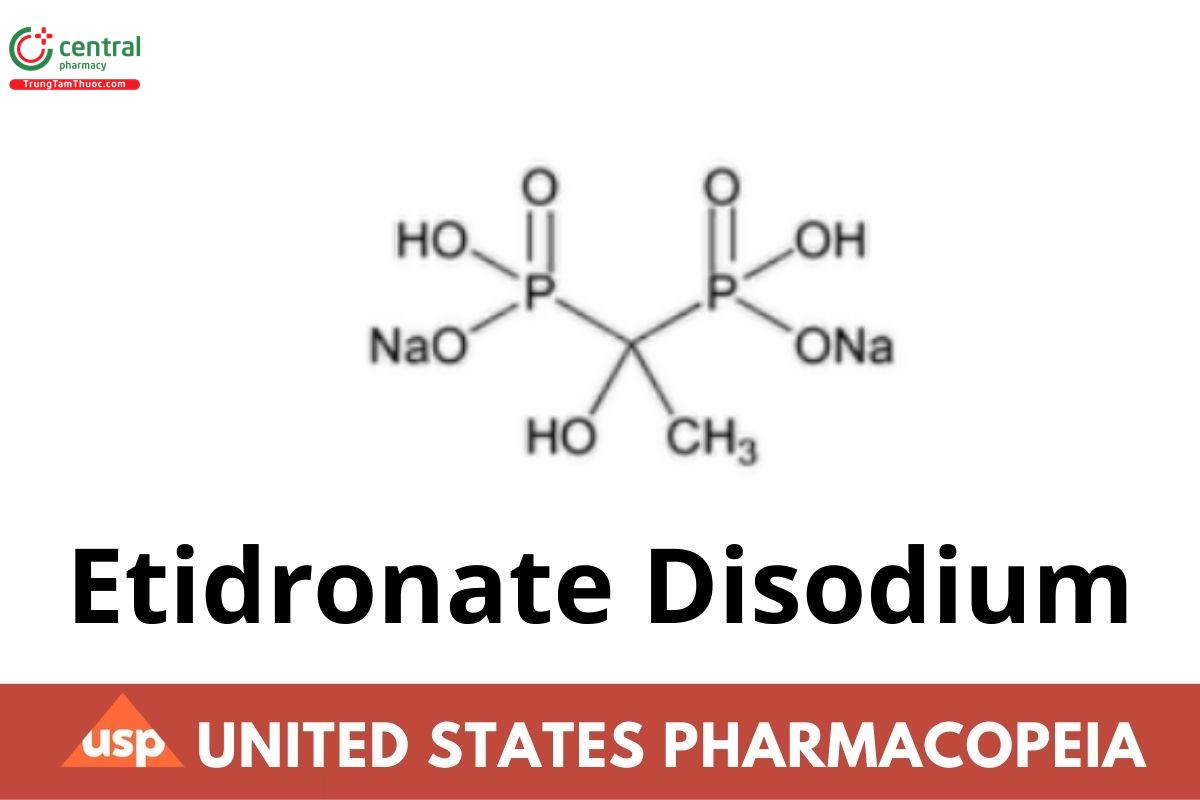
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Etidronate Disodium contains NLT 97.0% and NMT 101.0% of etidronate disodium (C2H6Na2O7P2), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy (ERR 1-Oct-2020)
Standard: Recrystallize USP Etidronate Disodium RS from water and dry it at 105° for 1 h.
Sample: Recrystallize the sample from water and dry it at 105° for 1 h.
Analysis and Acceptance criteria: The spectra of trifluorovinyl chloride polymer and Mineral oil dispersions of the Sample, separately prepared, exhibit maxima in the regions of 4000–1350 cm−1 and 1350–450 cm−1, respectively, only at the same wavelengths as those of similar preparations of the Standard. The analysis and acceptance criteria described in 〈197A〉 may also be used.
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Sodium: Meets the requirements of test A
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: 35–40 mM ammonium nitrate solution in water. Adjust with dilute ammonium hydroxide to a pH of 7.0.
Standard solution: 0.73–0.75 mg/mL of USP Etidronic Acid Monohydrate RS in 1 N sodium hydroxide VS and Mobile phase (1:150)
Sample solution: 0.84–0.86 mg/mL of Etidronate Disodium in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Refractive index
Column: 4.6-mm × 15-cm; 10-μm packing L23
Temperatures
Column: 32°
Detector: 32°
Flow rate: 1.5 mL/min
Injection volume: 20 μL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 1.5% for replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of etidronate disodium (C2H6Na2O7P2) in the portion of Etidronate Disodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Etidronic Acid Monohydrate RS in the Standard solution (mg/mL)
CU = concentration of Etidronate Disodium in the Sample solution (mg/mL)
Mr1 = molecular weight of etidronate disodium, 249.99
Mr2 = molecular weight of etidronic acid monohydrate, 224.04
Acceptance criteria: 97.0%–101.0% on the anhydrous basis
4 IMPURITIES
Limit of Phosphite
Solution A: 0.65 mg/mL of anhydrous sodium carbonate and 0.40 mg/mL of sodium bicarbonate in water
Solution B: 4.68 mg/mL of anhydrous sodium carbonate and 2.89 mg/mL of sodium bicarbonate in water
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 6.0 | 100 | 0 |
| 6.1 | 0 | 100 |
| 8.0 | 0 | 100 |
| 8.1 | 100 | 0 |
| 15 | 100 | 0 |
Suppressor regenerant solution: 12.5 mM sulfuric acid. [Note—This solution is needed only if the chemical suppression option is used.]
Standard solution: Equivalent to 0.016 mg/mL of dibasic sodium phosphite on the anhydrous basis from USP Etidronate Disodium Related
Compound A RS and 0.015 mg/mL of dibasic sodium phosphate in Solution A. [Note—USP Etidronate Disodium Related Compound A RS is sodium phosphite dibasic pentahydrate.]
Sample solution: 5.0 mg/mL of Etidronate Disodium in Solution A
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Conductivity
Columns
Guard: 4-mm × 50-mm; packing L61. [Note—Use either a 4-mm anion self-regenerating suppressor or a suitable chemical suppressor.]
Analytical: 4-mm × 25-cm; packing L61
Flow rate: 1.0 mL/min. [Note—When a chemical suppressor is used, the flow rate is 3–5 mL/min for the Suppressor regenerant solution.]
Injection volume: 20 μL
System suitability
Sample: Standard solution
[Note—The elution order is phosphite, followed by phosphate.]
Suitability requirements
Resolution: NLT 2.5 between phosphite and phosphate
Relative standard deviation: NMT 10% for each peak, for replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of phosphite, determined as monobasic sodium phosphite, in the portion of Etidronate Disodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of phosphite from the Sample solution
rS = peak response of phosphite from the Standard solution
CS = concentration of USP Etidronate Disodium Related Compound A RS on the anhydrous basis in the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Mr1 = molecular weight of monobasic sodium phosphite, 103.98
Mr2 = molecular weight of dibasic sodium phosphite, 125.96
Acceptance criteria: NMT 1.0% of phosphite, determined as monobasic sodium phosphite
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: 10 mg/mL
Acceptance criteria: 4.2–5.2
Water Determination 〈921〉, Method I, Method Ic
Sample solution: 100 mg of finely powdered Etidronate Disodium in 10 mL of a mixture of glacial acetic acid and formamide (1:1)
Acceptance criteria: NMT 5.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Etidronate Disodium RS
USP Etidronate Disodium Related Compound A RS
Sodium phosphite dibasic pentahydrate.
Na2HPO3 · 5H2O 216.04
USP Etidronic Acid Monohydrate RS
Phosphonic acid, (1-hydroxyethylidene)bis-, monohydrate
C2H8O7P2 · H2O 224.04


