Ethylparaben Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
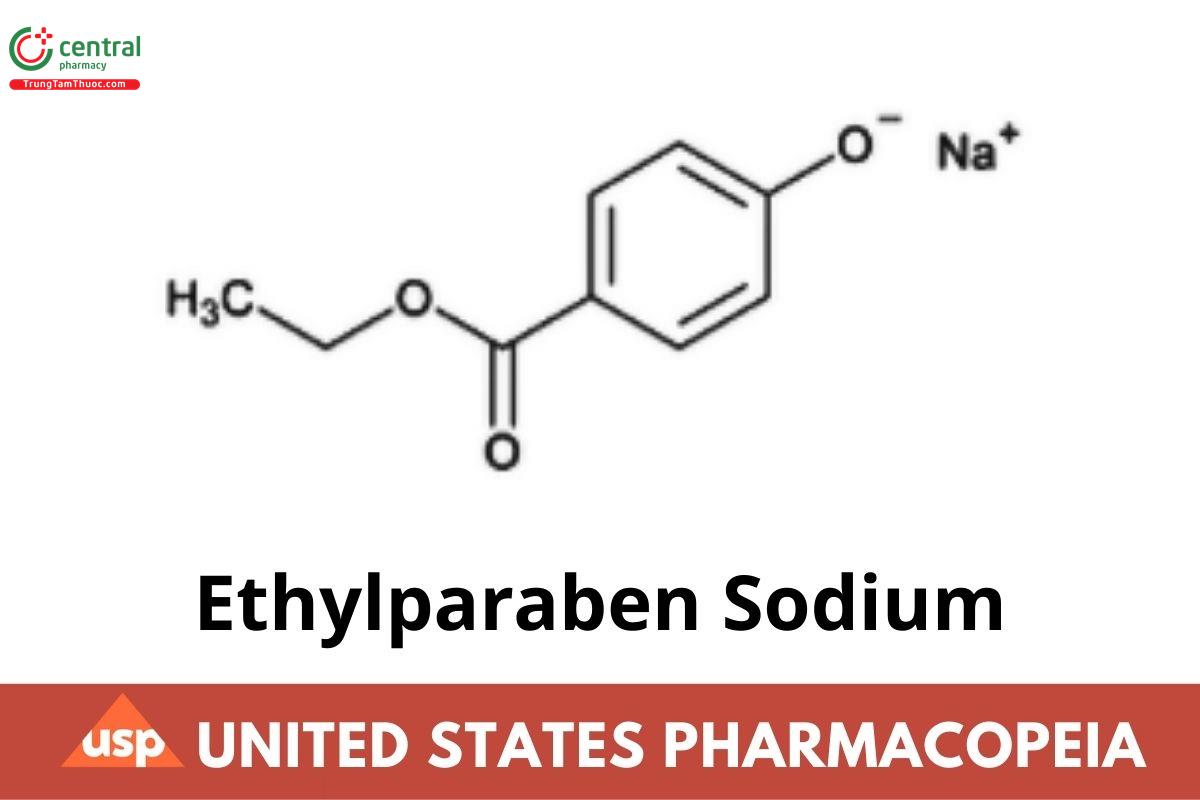
C9H9NaO3 188.2
Benzoic acid, 4-hydroxy-, methyl ester, sodium salt;
Ethyl p-hydroxybenzoate, sodium salt;
Sodium 4-ethoxycarbonylphenolate CAS RN®: 35285-68-8.
1 DEFINITION
Ethylparaben Sodium contains NLT 95.0% and NMT 102.0% of ethylparaben sodium (C9H9NaO3), calculated on the anhydrous basis.
2 IDENTIFICATION
2.1 A.
Standard: 0.5 g of USP Ethylparaben RS
Sample: 0.5 g of Ethylparaben Sodium
Analysis: Dissolve the Sample in 5 mL of water, acidify with hydrochloric acid, and filter the resulting precipitate. Wash the precipitate with water, and dry under vacuum at 80° for 2 h.
Acceptance criteria: The IR absorption spectrum of a Mineral oil dispersion of the Sample exhibits maxima only at the same wavelengths as those of a similar preparation of the Standard.
2.2 B.
Sample solution: Ignite 0.3 g of Ethylparaben Sodium, cool, and dissolve the residue in about 3 mL of 3 N hydrochloric acid.
Acceptance criteria: A platinum wire dipped in the Sample solution imparts an intense, persistent yellow color to a nonluminous flame.
3 ASSAY
3.1 PROCEDURE
Mobile phase: Methanol and a 6.8-g/L solution of potassium dihydrogen phosphate (65:35)
System suitability solution: 5.0 µg/mL each of p-hydroxybenzoic acid, USP Methylparaben RS, and USP Ethylparaben RS in Mobile phase
Standard solution: Dissolve 50.0 mg of USP Ethylparaben RS in 2.5 mL of methanol, and dilute with Mobile phase to 50.0 mL. Dilute 10.0 mL of this solution with Mobile phase to 100.0 mL.
Sample solution: Dissolve 50.0 mg of Ethylparaben Sodium in 2.5 mL of methanol, and dilute with Mobile phase to 50.0 mL. Dilute 10.0 mL of
this solution with Mobile phase to 100.0 mL.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 272 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1.3 mL/min
Injection volume: 10 µL
Run time: About 4 times the retention time of the ethylparaben peak
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The retention time of ethylparaben is about 2.9 min; the relative retention times for p-hydroxybenzoic acid, methylparaben, and ethylparaben are about 0.5, 0.8, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between methylparaben and ethylparaben peaks, System suitability solution
Relative standard deviation: NMT 0.85% for six injections, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ethylparaben sodium in the portion of Ethylparaben Sodium taken:
Result = (rU/rS) x (CS/CU) x (Mr1/Mr2) x P
rU = peak area of ethylparaben from the Sample solution
rS = peak area of ethylparaben from the Standard solution
CS = concentration of USP Ethylparaben RS in the Standard solution
CU = concentration of Ethylparaben Sodium in the Sample solution
Mr1 = molecular weight of ethylparaben sodium, 188.2
Mr2 = molecular weight of ethylparaben, 166.17
P = labeled purity of USP Ethylparaben RS expressed as a percentage
Acceptance criteria: 95.0%-102.0% on the anhydrous basis
4 IMPURITIES
4.1 RELATED COMPOUNDS
Mobile phase, System suitability solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: Dilute 1.0 mL of the Sample solution with Mobile phase to 20.0 mL. Dilute 1.0 mL of this solution with Mobile phase to 10.0 mL.
System suitability
Sample: System suitability solution
[NOTE-The retention time of ethylparaben is about 2.9 min; the relative retention times for p-hydroxybenzoic acid, methylparaben, and ethylparaben are about 0.5, 0.8, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between methylparaben and ethylparaben peaks
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria
p-Hydroxybenzoic acid: The peak area in the Sample solution, multiplied by 1.4 to correct for the calculation of content, is NMT 6 times the area of the principal peak in the Standard solution; NMT 3.0%.
Unspecified impurities: The peak area of each impurity in the Sample solution is NMT the area of the principal peak in the Standard solution; NMT 0.5%.
Total impurities: The total peak area for all unspecified impurities in the Sample solution is NMT twice the area of the principal peak in the
Standard solution; NMT 1.0%.
4.2 CHLORIDE AND SULFATE, Chloride (221)
Sample: 0.2 g
Control solution: 0.10 mL of 0.020 N hydrochloric acid
Acceptance criteria: 0.035%; the Sample shows no more chloride than the Control solution.
4.3 CHLORIDE AND SULFATE, Chloride (221)
Sample: 0.2 g
Control solution: 0.10 mL of 0.020 N hydrochloric acid
Acceptance criteria: 0.035%; the Sample shows no more chloride than the Control solution.
4.4 CHLORIDE AND SULFATE, Sulfate (221)
Sample: 1.0 g
Control solution: 0.31 mL of 0.020 N sulfuric acid
Acceptance criteria: 0.030%; the Sample shows no more sulfate than the Control solution.
5 SPECIFIC TESTS
PH (791)
Sample solution: 1 mg/mL
Acceptance criteria: 9.5-10.5
WATER DETERMINATION, Method (921): NMT 5.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
USP REFERENCE STANDARDS (11)
USP Ethylparaben RS
USP Methylparaben RS


