Ethylparaben
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
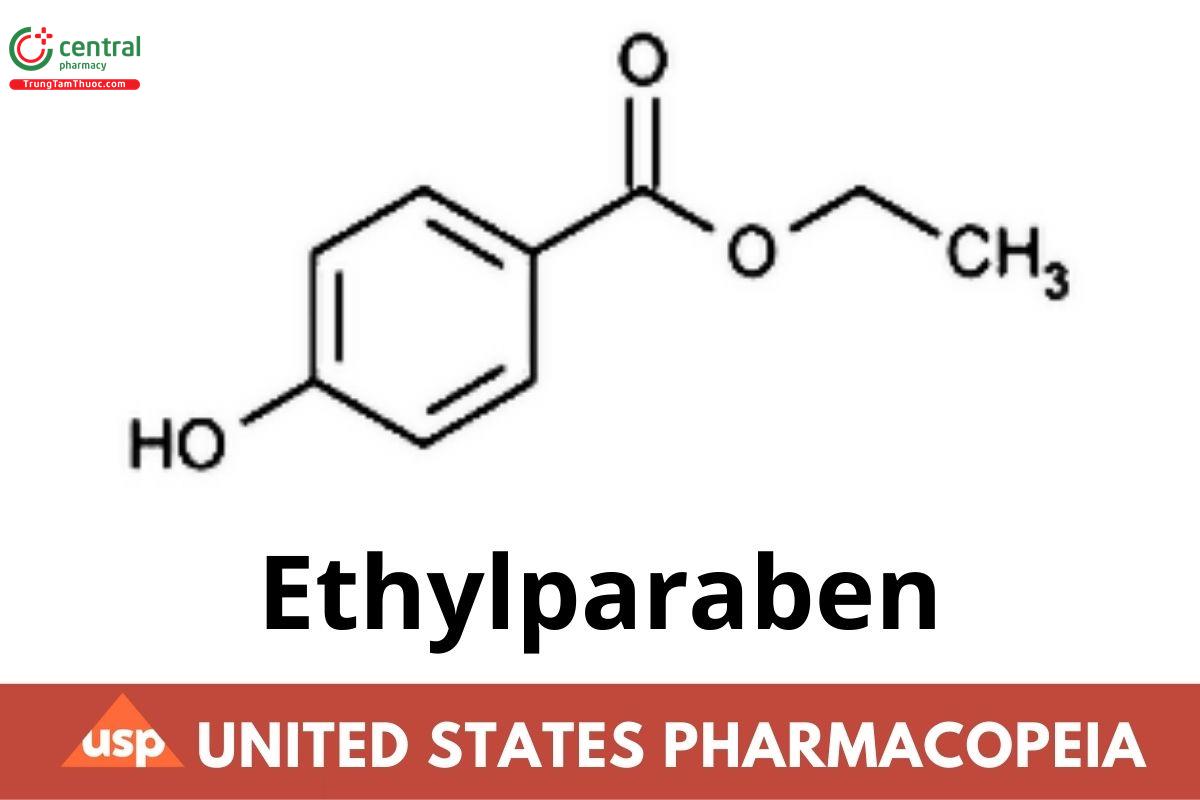
C9H10O3 166.17
Benzoic acid, 4-hydroxy-, ethyl ester;
Ethyl p-hydroxybenzoate CAS RN: 120-47-8.
1 DEFINITION
Ethylparaben contains NLT 98.0% and NMT 102.0% of C,H, 0 103
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M (CN 1-M-2020)
B. MELTING RANGE OR TEMPERATURE (741): 115°-118°
3 ASSAY
PROCEDURE
Mobile phase, Sample solution, Standard solution B, and Chromatographic system: Proceed as described in the procedure for Related Substances.
System suitability
Sample: Standard solution B
Suitability requirements
Relative standard deviation: NMT 0.85% for 6 injections
Analysis
Samples: Sample solution and Standard solution B
Calculate the percentage of Ethylparaben in the Sample solution:
Result = P × (ru × Cs )/(rs × Cu )
P = labeled purity of USP Ethylparaben RS expressed as a percentage
ru = peak area of ethylparaben from the Sample solution
Cs = concentration of ethylparaben in Standard solution B
rs = peak area of ethylparaben from Standard solution B
Cu = concentration of Ethylparaben in the Sample solution
Acceptance criteria: 98.0%–102.0%
4 IMPURITIES
4.1 Inorganic Impurities
RESIDUE ON IGNITION (281): NMT 0.1%, determined on 1.0 g
4.2 Organic Impurities
PROCEDURE: RELATED SUBSTANCES
Mobile phase: Methanol and a 6.8-g/L solution of potassium dihydrogen phosphate (65:35 v/v)
Sample solution: Dissolve 50.0 mg of Ethylparaben in 2.5 ml. of methanol, and dilute with Mobile phase to 50.0 mL. Dilute 10.0 mL of this solution with Mobile phase to 100.0 mL.
Standard solution A: 5.0 µg/ml. each of p-hydroxy benzoic acid, USP Methylparaben RS, and USP Ethylparaben RS in Mobile phase
Standard solution B: Dissolve 50.0 mg of USP Ethylparaben RS in 2.5 mL of methanol, and dilute with Mobile phase to 50.0 mL. Dilute 10.0 mL of this solution with Mobile phase to 100.0 mL.
Standard solution C: Dilute 1.0 mL of the Sample solution with Mobile phase to 20.0 mL. Dilute 1.0 mL of this solution with Mobile phase to 10.0 mL.
Chromatographic system
(See Chromatography (621) System Suitability)
Mode: LC
Detector: UV 272 nm
Column: 4.6 mm x 15-cm; 5-um packing L1
Flow rate: 1.3 mL/min
Injection size: 10 µL
Run time: About 4 times the retention time of ethylparaben
System suitability
Sample: Standard solution A
[NOTE-The retention time of ethylparaben is about 3.0 min; the relative retention times for p-hydroxybenzoic acid, ethylparaben, and methylparaben are about 0.5, 1.0, and 0.8, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the methylparaben and ethylparaben peaks
Analysis
Samples: Sample solution and Standard solution C
[NOTE-Disregard any limit that is 0.2 times the area of the principal peak in the chromatogram obtained with Standard solution C (0.1%).]
Acceptance criteria
p-Hydroxybenzoic acid: The peak area in the Sample solution, multiplied by 1.4 to correct for the calculation of content, is NMT the area. of the principal peak in Standard solution C (0.5%).
Unspecified impurities: The peak area of each impurity in the Sample solution is NMT the area of the principal peak in Standard solution C (0.5%).
Total impurities: The total peak area for all impurities in the Sample solution is NMT twice the area of the principal peak in Standard solution C (1.0%).
5 SPECIFIC TESTS
COLOR OF SOLUTION
Sample solution: 100 mg/mL in alcohol
Comparison solution: Mix 2.4 mL of ferric chloride CS, 1.0 1.0 mL of cobaltous chloride CS, and 0.4 mL of cupric sulfate CS with 0.3 N hydrochloric acid to make 10 mL. Dilute 5 mL of this solution with 0.3 N hydrochloric acid to make 100 ml. [NOTE-Prepare and use this solution immediately.]
Analysis
Samples: Alcohol, Sample solution, and Comparison solution
Make the comparison by viewing the solutions downward in matched color-comparison tubes against a white surface (see Color and Achromicity (631)),
Acceptance criteria: The Sample solution is clear and not more intensely colored than alcohol or the Comparison solution.
Sample solution: To 2 mL of Sample solution prepared in the test for Color of Solution, add 3 mL of alcohol, 5 mL of carbon dioxide-free water, and 0.1 mL of bromocresol green TS.
Analysis: Titrate with 0.10 N sodium hydroxide.
Acceptance criteria: NMT 0.1 ml. is required to produce a blue color.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers.
USP REFERENCE STANDARDS (11)
USP Ethylparaben RS
USP Methylparaben RS


