Ethyl Oleate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
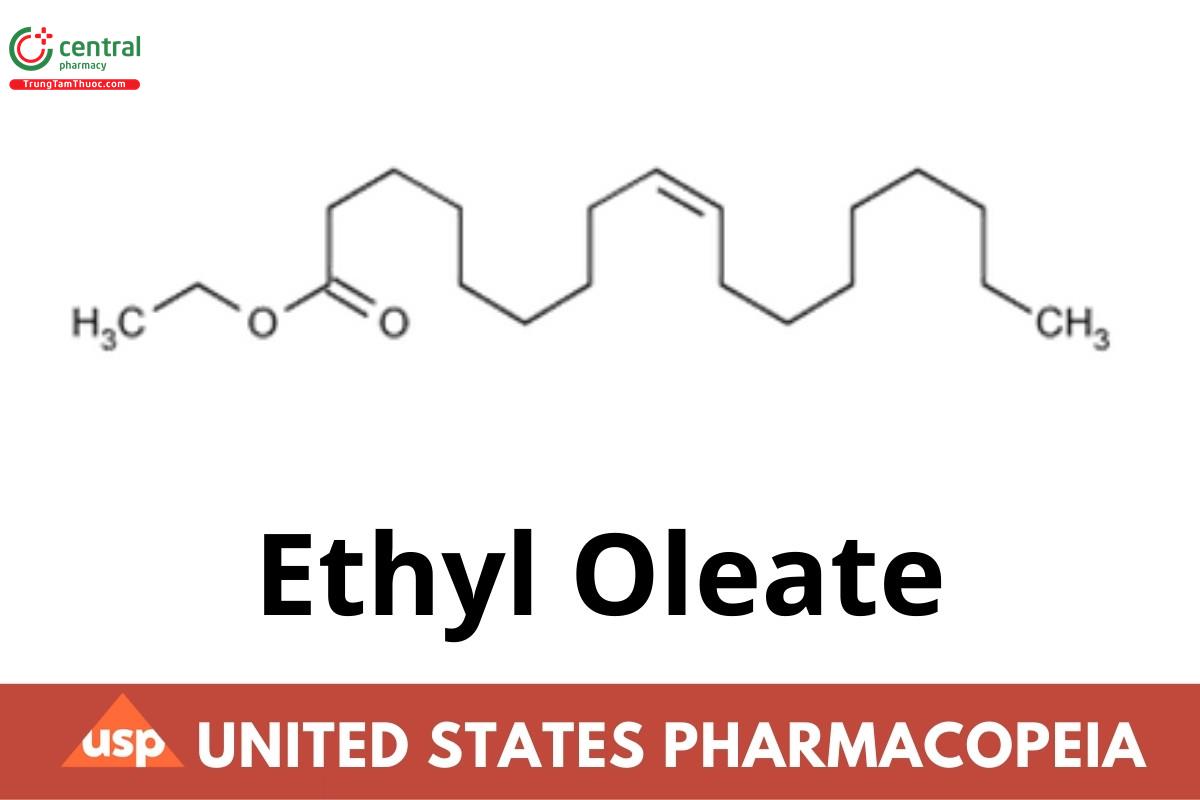
Ethyl Oleate consists of esters of ethyl alcohol and high molecular weight fatty acids, principally oleic acid. It contains NLT 65.0% of ethyl (Z)-9- octadecenoate (C20H38O2). It may contain suitable stabilizers.
2 IDENTIFICATION
2.1 A. PRESENCE OF ESTER
Analysis 1: Proceed as directed in Fats and Fixed Oils, Saponification Value 〈401〉. Acceptance criteria: 177–188
Analysis 2: Proceed as directed in Fats and Fixed Oils, Acid Value〈401〉. Acceptance criteria: NMT 0.5
2.2 B. CHROMATOGRAPHIC IDENTITY
Analysis: Proceed as directed in the Assay.
Acceptance criteria: The retention time of the major peak of the Sample solution corresponds to the ethyl oleate peak of the System suitability solution.
3 ASSAY
3.1 PROCEDURE
System suitability solution: 5 mg/mL of USP Ethyl Oleate RS, 1.2 mg/mL of USP Ethyl Palmitate RS, 1.2 mg/mL of USP Ethyl Linoleate RS, and 0.5 mg/mL of USP Ethyl Stearate RS in n-hexane
Sample solution: 5 mg/mL of Ethyl Oleate in n-hexane
3.1.1 Chromatographic system
(See Chromatography 〈621〉, System Suitability.) Mode: GC
Detector: Flame ionization
Column: 0.25-mm × 25-m capillary; bonded with a 0.2-µm layer of phase G16
Temperatures
Detector: 270°
Injection port: 250°
Column: See temperature program shown in Table 1.
Table 1
Carrier gas: Hydrogen Flow rate: 0.7 mL/min Injection volume: 1.0 µL
Injection type: Split injection; split ratio is 200:1 Run time: 23–24 min
3.1.2 System suitability
Sample: System suitability solution
[NOTE—See Table 2 for the relative retention times.]
Table 2
Suitability requirements
Resolution: NLT 2.0 between the ethyl stearate and ethyl oleate peaks
3.1.3 Analysis
Samples: System suitability solution and Sample solution
Identify each ethyl ester (ethyl palmitate, ethyl stearate, ethyl oleate, or ethyl linoleate) peak in the Sample solution based on that in the System suitability solution.
Calculate the percentage of each ethyl ester (ethyl palmitate, ethyl stearate, ethyl oleate, or ethyl linoleate) in the portion of Ethyl Oleate taken:
Result = (rU/rT) × 100
rU = peak response of each ethyl ester (ethyl palmitate, ethyl stearate, ethyl oleate, or ethyl linoleate) from the Sample solution
rT = sum of all the peak responses excluding peak responses due to solvent from the Sample solution
Acceptance criteria: Disregard peaks that are less than 0.05% for any unspecified impurities, and any peaks due to solvent. Ethyl Oleate exhibits the composition profiles shown in Table 3 below.
4 IMPURITIES
TOTAL ASH
Sample: 2.0 g
Analysis: Heat a silica or platinum crucible to redness for 30 min, allow to cool in a desiccator, and weigh. Transfer the Sample to the crucible. Dry at 100°–105° for 1 h and ignite to constant weight in a muffle furnace at 600 ± 25°, allowing the crucible to cool in a desiccator after each ignition. Flames should not be produced at any time during the procedure. If after prolonged ignition the ash still contains black particles, take up with hot water, pass through an ashless filter paper, and ignite the residue and the filter paper. Combine the filtrate with the ash, carefully evaporate to dryness, and ignite to constant weight.
Acceptance criteria: NMT 0.1%
5 SPECIFIC TESTS
FATS AND FIXED OILS, Iodine Value 〈401〉: 75–85
FATS AND FIXED OILS, Peroxide Value 〈401〉: NMT 10.0
WATER DETERMINATION 〈921〉: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
LABELING: Label it to indicate whether oleic acid is derived from vegetable, animal, or synthetic sources. Indicate the names and amounts of any added stabilizers.
USP REFERENCE STANDARDS 〈11〉
USP Ethyl Linoleate RS USP Ethyl Oleate RS USP Ethyl Palmitate RS USP Ethyl Stearate RS


