Ethyl Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
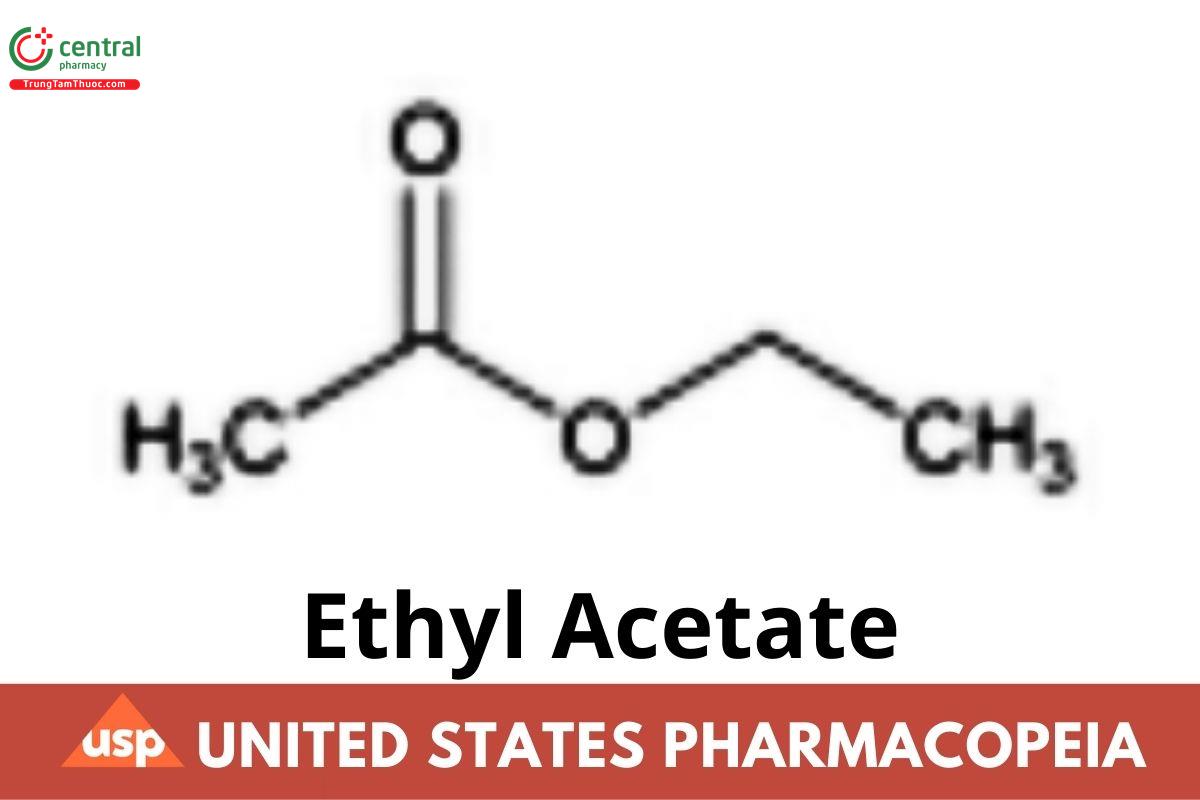
C4H8O2 88.11
Acetic acid, ethyl ester;
Ethyl acetate CAS RN®: 141-78-6.
1 DEFINITION
Ethyl Acetate contains NLT 98.0% and NMT 102.0% of ethyl acetate (C4H8O2).
2 IDENTIFICATION
Change to read:
Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F
3 ASSAY
Procedure
Diluent: N,N-dimethylacetamide
System suitability solution: 2.0 mg/mL of USP Ethyl Acetate RS and 20 µg/mL of USP Methyl Ethyl Ketone RS in Diluent Standard solution: 2.0 mg/mL of USP Ethyl Acetate RS in Diluent
Sample solution: 2.0 mg/mL of Ethyl Acetate in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 60-m; coated with a 1.8-µm lm of phase G43
Temperatures
Detector: 250°
Injection port: 210°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 40 | - | 40 | 15 |
| 40 | 12 | 200 | 2 |
Carrier gas: Helium
Flow rate: 3.0 mL/min
Injection volume: 1 µL
Injection type: Split injection, split ratio 30:1
Run time: 30.3 min
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for methyl ethyl ketone and ethyl acetate are 0.97 and 1.0, respectively.] Suitability requirements
Resolution: NLT 2.0 between the methyl ethyl ketone and ethyl acetate peaks, System suitability solution
Tailing factor: NMT 1.5 for the ethyl acetate peak, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ethyl acetate (C4H8O2) in the portion of Ethyl Acetate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak area of ethyl acetate from the Sample solution
rS = peak area of ethyl acetate from the Standard solution
CS = concentration of USP area of ethyl acetate RS in the Standard solution (mg/mL)
CU = concentration of area of ethyl acetate in the Sample solution (mg/mL)
P = labeled purity of USP Ethyl Acetate RS
Acceptance criteria: 98.0%–102.0%
4 IMPURITIES
Limit of Nonvolatile Residue
Sample: Ethyl Acetate
Analysis: Evaporate the Sample in a tared porcelain dish on a steam bath, and dry at 105° for 1 h. Acceptance criteria: NMT 0.02%
Change to read:
Chromatographic Purity
Diluent and Chromatographic system: Proceed as directed in the Assay.
System suitability solution: 0.16 mg/mL each of acetaldehyde and methanol, 160 mg/mL of Ethyl Acetate, and 1.6 mg/mL of USP Methyl Ethyl Ketone RS in Diluent
Sensitivity solution: 0.08 mg/mL each of acetaldehyde, USP Ethyl Acetate RS, and USP 1-Ethoxy-2-methylpropane RS in Diluent Methyl compounds identication solution: 0.16 mg/mL each of methanol, methyl acetate, and methyl isobutyrate in Diluent Standard solution: 0.16 mg/mL each of acetaldehyde, USP Ethyl Acetate RS, and USP 1-Ethoxy-2-methylpropane RS in Diluent Sample solution: 160 mg/mL of Ethyl Acetate in Diluent
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[Note—The relative retention times for acetaldehyde, methanol, methyl ethyl ketone, ethyl acetate, and 1-ethoxy-2-methylpropane are 0.29, 0.31, 0.97, 1.0, and 1.1, respectively.]
Suitability requirements
Resolution: NLT 2.0 between the acetaldehyde and methanol peaks; NLT 2.0 between the methyl ethyl ketone and ethyl acetate peaks, System suitability solution
Signal-to-noise ratio: NLT 20 for the acetaldehyde, ethyl acetate, and 1-ethoxy-2-methylpropane peaks, Sensitivity solution Tailing factor: NMT 1.5 for the acetaldehyde, ethyl acetate, and 1-ethoxy-2-methylpropane peaks, Standard solution Relative standard deviation: NMT 5.0% for acetaldehyde, ethyl acetate, and 1-ethoxy-2-methylpropane, Standard solution Analysis
Samples: Methyl compounds identification solution, Standard solution, and Sample solution
Calculate the percentage of acetaldehyde and 1-ethoxy-2-methylpropane in the portion of Ethyl Acetate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak area of acetaldehyde or 1-ethoxy-2-methylpropane from the Sample solution
rS = peak area of acetaldehyde or 1-ethoxy-2-methylpropane from the Standard solution
CS = concentration of acetaldehyde or USP 1-Ethoxy-2-methylpropane RS in the Standard solution (mg/mL)
CU = concentration of area of ethyl acetate in the Sample solution (mg/mL)
Identify the methanol, methyl acetate, and methyl isobutyrate peaks in the Sample solution based on those in the Methyl compounds identification solution.
Calculate the content of methyl compounds in the portion of Ethyl Acetate taken:
Result = (rT/rS) × F
rT = sum of the peak areas of methanol, methyl acetate, and methyl isobutyrate from the Sample solution
rS = peak area of ethyl acetate from the Standard solution
F = limit of methyl compounds, in percentage, 0.1
Acceptance criteria
Acetaldehyde: NMT 0.1%
1-Ethoxy-2-methylpropane: NMT 0.1%
Methyl compounds: NMT 0.1%
Other impurities: NMT 0.3%; the sum of all other peaks areas in the chromatogram of the Sample solution, excluding the ethyl acetate, specified impurities, and solvent peaks areas, is not greater than three times the ethyl acetate peak area of the Standard solution.
5 SPECIFIC TESTS
Acidity
Sample solution: 2.0 mL of Ethyl Acetate in 10 mL of neutralized alcohol
Analysis: Add 2 drops of phenolphthalein TS to the Sample solution. Neutralize with 0.10 N sodium hydroxide.
Acceptance criteria: NMT 0.10 mL of 0.10 N sodium hydroxide is required.
Readily Carbonizable Substances Test 〈271〉
Sample: 2 mL of Ethyl Acetate
Analysis: Add the Sample carefully to 10 mL of sulfuric acid to form separate layers.
Acceptance criteria: No dark zone is developed within 15 min.
Specific Gravity 〈841〉: 0.894–0.898
Bacterial Endotoxins Test 〈85〉: If labeled for use in preparing parenteral dosage forms, it also meets the following requirements. The level of bacterial endotoxins is such that the requirement in the relevant dosage form monographs(s) in which Ethyl Acetate is used can be met. Where the label states that Ethyl Acetate must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Ethyl Acetate is used can be met.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and avoid exposure to excessive heat.
Labeling: Where Ethyl Acetate is intended for use in the manufacture of injectable dosage forms, it is so labeled. Where Ethyl Acetate must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled.
USP Reference Standards 〈11〉
USP Ethyl Acetate RS
USP 1-Ethoxy-2-methylpropane RS
USP Methyl Ethyl Ketone RS


