Ether
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
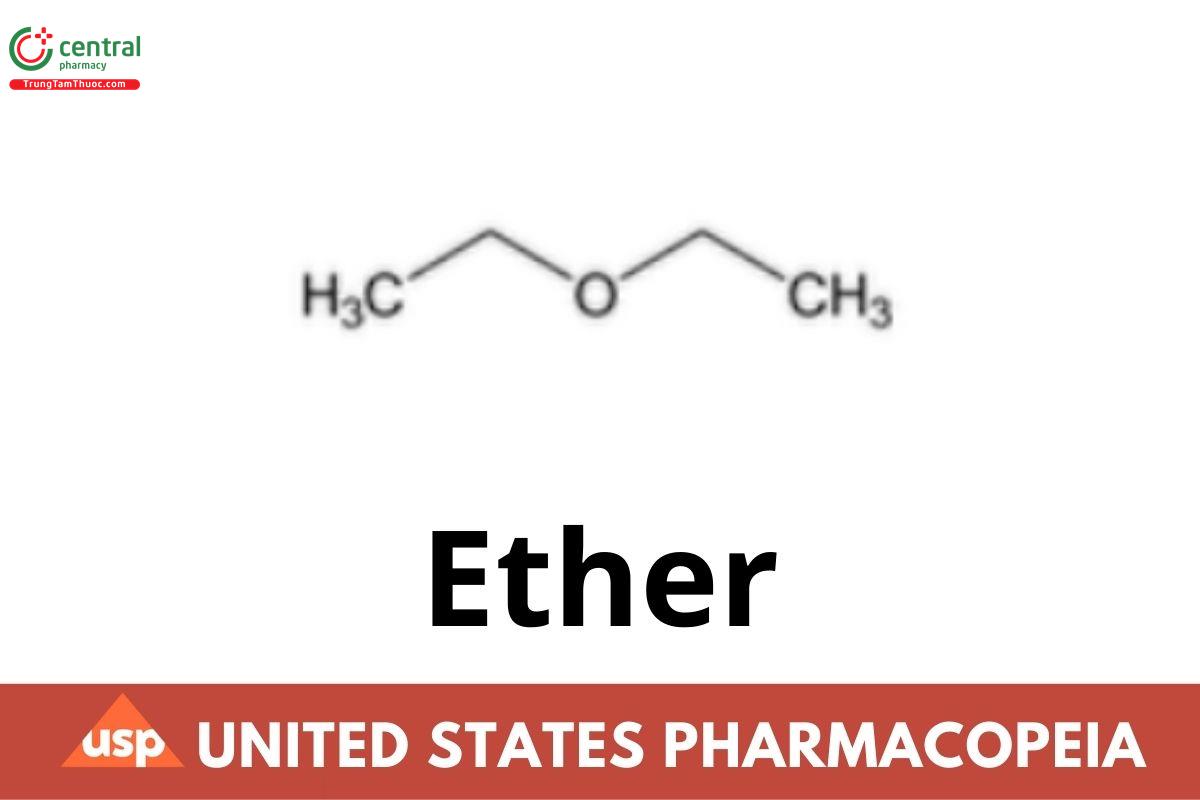
Ether contains NLT 96.0% and NMT 98.0% of ether (C4H10O), the remainder consisting of alcohol and water.
[Caution—Ether is highly volatile and flammable. Its vapor, when mixed with air and ignited, may explode.]
[Note—Ether to be used for anesthesia must be preserved in tight containers of NMT 3-kg capacity, and is not to be used for anesthesia if it has been removed from the original container longer than 24 h. Ether to be used for anesthesia may, however, be shipped in larger containers for repackaging in containers as directed above, provided the ether at the time of repackaging meets the requirements of the tests of this Pharmacopeia.]
2 IMPURITIES
Limit of Nonvolatile Residue
Sample: 50 mL
Analysis: Allow the Sample to evaporate spontaneously from a tared dish, and dry at 105° for 1 h.
Acceptance criteria: 0.003%; the weight of the residue is NMT 1 mg.
Limit of Low-Boiling Hydrocarbons
Standard solution: Anhydrous ethyl ether, previously tested for absence of hydrocarbons, as directed for Analysis, and pentane (99.8:0.2)
Sample solution: Ether
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 2-mm × 3.7-m stainless steel; with 30% phase G22 on 30- to 60-mesh support S1D
Temperatures
Column: 80°
Injection port: 230°
Detector: 250°
Flow rate: 30 mL/min
Carrier gas: Nitrogen
Injection volume: 1 μL
Analysis
Samples: Standard solution and Sample solution
[Note—The retention times are about 2.3 min for isopentane, 2.7 min for pentane, and 4.3 min for 2-methylpentane.]
Measure the total area under the hydrocarbon peaks in the Sample solution.
Acceptance criteria: 0.2%; the total area of the hydrocarbon peaks does not exceed that of the peak for pentane in the Standard solution.
Limit of Aldehyde
Solution A: A mixture of 1 mL of alkaline mercuric–potassium iodide TS and 17 mL of a saturated solution of sodium chloride
Sample: 20 mL
Analysis: Place the Sample in a glass-stoppered cylinder, and add 7 mL of Solution A. Insert the stopper in the cylinder, shake vigorously for 10 s, and then set aside for 1 min.
Acceptance criteria: The water layer shows no turbidity.
Limit of Peroxide
Solution A: Cool separately, in small beakers surrounded by crushed ice, 10 mL of 6 N hydrochloric acid and 10 mL of titanium tetrachloride.
Add the titanium tetrachloride dropwise to the chilled acid. Allow the mixture to stand at ice-bath temperature until all of the yellow solid dissolves, and dilute the solution with 6 N hydrochloric acid to 1000 mL.
Standard solution: 0.011 mg/mL of H O , prepared as follows. Pipet 25 mL of Hydrogen peroxide solution into a 1000-mL volumetric flask, and dilute with water to volume. Pipet 15 mL of this solution into a 1000-mL volumetric flask, and dilute with water to volume.
Sample solution: Ether
Instrumental conditions
Mode: Vis
Analytical wavelength: 410 nm
Cell: 1 cm
Analysis
Samples: Standard solution and Sample solution
To 50 mL of the Sample solution, in a separator, add 5.0 mL of Solution A. Shake vigorously, allow the layers to separate, and drain the lower layer into a glass-stoppered, 25-mL graduated cylinder. Dilute with water to 10.0 mL. Prepare a second solution by adding 5.0 mL of Solution A and 1.0 mL of Standard solution to a glass-stoppered, 25-mL graduated cylinder, and diluting with water to 10.0 mL. Use a suitable spectrophotometer to determine the colors.
Acceptance criteria: NMT 0.3 ppm; any yellow color in the Sample solution does not exceed that in the Standard solution.
3 SPECIFIC TESTS
Specific Gravity 〈841〉: 0.713–0.716, indicating 96.0%–98.0% of C H O
Acidity
Analysis: Exercise great care to avoid contamination from carbon dioxide when adding the Ether and titrating. To 10 mL of water in a glass-stoppered flask, add 0.10 mL of bromothymol blue TS and 0.010 N sodium hydroxide until a blue color persists after vigorous shaking. Add 25 mL of Ether, and shake briskly to mix the two layers. If no blue color remains, titrate with 0.010 N sodium hydroxide until the blue color is restored and persists for several min.
Acceptance criteria: 0.003% as CH COOH; NMT 0.80 mL of 0.010 N sodium hydroxide is required.
Water Determination, Method I〈921〉: NMT 0.5%; except where labeled as intended for anesthetic use, it contains NMT 0.2%.
4 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in partly filled, tight, light-resistant containers, at a temperature not exceeding 30° remote from fire.
Labeling: Where Ether is intended for anesthetic use, the label so states.


