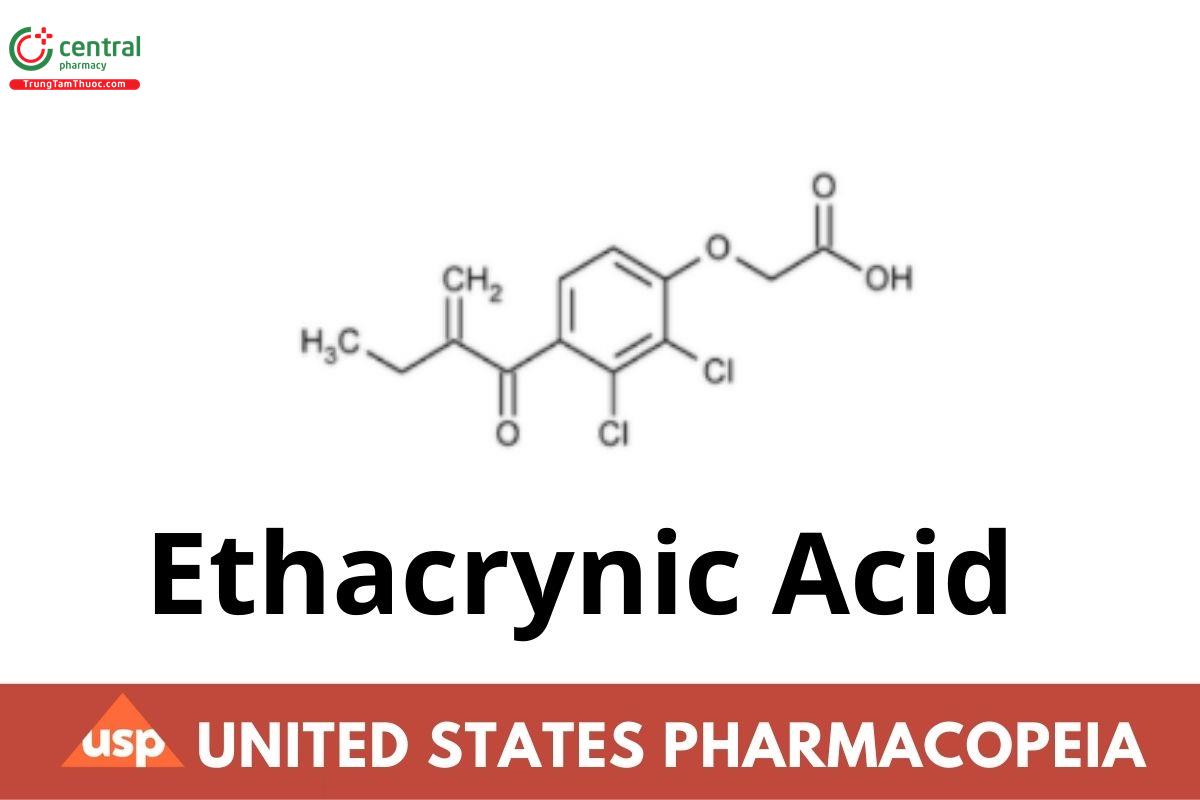
Ethacrynic Acid
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Ethacrynic Acid contains NLT 97.0% and NMT 102.0% of ethacrynic acid (C13H12Cl2O4), calculated on the dried basis.
[Caution—Use care in handling Ethacrynic Acid, because it irritates the skin, eyes, and mucous membranes.]
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M or 197A (USP 1-Dec-2021)
Delete the following:
B. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U
Analytical wavelength: 271 nm
Sample solution: 50 μg/mL in methanol
Acceptance criteria: Absorptivities, calculated on the dried basis, do not differ by more than 3.0%. (USP 1-Dec-2021)
Add the following:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP1-Dec-2021)
Delete the following:
C. Procedure
Sample solution: 12.5 mg/mL in 1 N sodium hydroxide
Analysis: Heat 2 mL of Sample solution for several min in a boiling water bath. Cool the solution, acidify with 0.25 mL of 18 N sulfuric acid, add 0.5 mL of chromotropic acid sodium salt solution (1 in 10), then add, cautiously, 2 mL of sulfuric acid TS.
Acceptance criteria: A deep violet color is produced. (USP 1-Dec-2021)
3 ASSAY
Change to read:
Procedure
Solution A: Triethylamine and water (1:100). Adjust with phosphoric acid to a pH of 6.8.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 75 | 25 |
| 15 | 75 | 25 |
| 18 | 45 | 55 |
| 20 | 75 | 25 |
Diluent: Acetonitrile and water (25:75)
Standard solution: 0.1 mg/mL of USP Ethacrynic Acid RS in Diluent. Sonicate to dissolve.
Sample solution: 0.1 mg/mL of Ethacrynic Acid in Diluent. Sonicate to dissolve.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm × 25-cm; 5-μm packing L1
Column temperature: 30°
Flow rate: 1.8 mL/min
Injection volume: 50 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.5
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ethacrynic acid (C13H12Cl2O4) in the portion of Ethacrynic Acid taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of ethacrynic acid from the Sample solution
rS = peak response of ethacrynic acid from the Standard solution
CS = concentration of USP Ethacrynic Acid RS in the Standard solution (mg/mL)
CU = concentration of Ethacrynic Acid in the Sample solution (mg/mL) (USP 1-Dec-2021)
Acceptance criteria: 97.0%–102.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Add the following:
Organic Impurities
Solution A, Solution B, Diluent, and Chromatographic system: Proceed as directed in the Assay.
Mobile phase: See Table 2.
Table 2
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 75 | 25 |
| 20 | 75 | 25 |
| 45 | 45 | 55 |
| 50 | 45 | 55 |
| 51 | 75 | 25 |
| 60 | 75 | 25 |
System suitability solution: 1 mg/mL of USP Ethacrynic Acid RS and 1.5 μg/mL of USP Ethacrynic Acid Related Compound A RS in Diluent.
Sonicate to dissolve.
Sensitivity solution: 0.3 μg/mL of USP Ethacrynic Acid RS in Diluent
Standard solution: 3 μg/mL of USP Ethacrynic Acid RS in Diluent
Sample solution: 1 mg/mL of Ethacrynic Acid in Diluent. Sonicate to dissolve.
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[Note—See Table 3 for the relative retention times.]
Suitability requirements
Resolution: NLT 2.0 between the ethacrynic acid related compound A and ethacrynic acid peaks, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Ethacrynic Acid taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of ethacrynic acid from the Standard solution
CS = concentration of USP Ethacrynic Acid RS in the Standard solution (mg/mL)
CU = concentration of Ethacrynic Acid in the Sample solution (mg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. The reporting threshold is 0.03%.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
Ethacrynic acid dimethylamino analoga | 0.26 | 1.49 | 0.15 |
Ethacrynic acid related compound A | 0.74 | 1.61 | 0.15 |
| Ethacrynic acid | 1.0 | - | - |
Ethacrynic acid trichloro analogb | 1.56 | 1.18 | 0.15 |
| Ethacrynic acid pyrane dimerc | 3.47 | 0.73 | 0.3 |
| Any unspecified impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 1.0 |
a {2,3-Dichloro-4-[2-{(dimethylamino)methyl}butanoyl]phenoxy}acetic acid hydrochloride.
b {2,3-Dichloro-4-[2-(chloromethyl)butanoyl]phenoxy}acetic acid.
c {4-[2-(4-(Carboxymethoxy)-2,3-dichlorobenzoyl]-2,5-diethyl-3,4-dihydro-2H-pyran-6-yl}-2,3-dichlorophenoxy)acetic acid.▲ (USP 1-Dec-2021)
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry at a pressure not exceeding 5 mm of mercury at 60° for 2 h.
Acceptance criteria: NMT 0.25%
Delete the following:
Equivalent Weight
Sample solution: 400 mg in 100 mL of methanol. Add 5 mL of water.
Analysis: Titrate with 0.1 N sodium hydroxide VS, using a calomel–glass electrode system or other appropriate electrode (see Titrimetry〈541〉). Perform a blank determination, and make any necessary correction. Calculate the equivalent weight on the dried basis.
Acceptance criteria: Between 294 and 309 (USP 1-Dec-2021)
Delete the following:
Toluene Extractives
Sample solution: 1 g into a glass-stoppered, 100-mL cylinder. Add 50 mL of sodium sulfite solution (2 in 25), and agitate until the solid dissolves. Allow to stand for 20 min, and add 5 mL of hydrochloric acid.
Analysis: Divide the solution between two centrifuge tubes, each of which contains 15 mL of toluene. Close each tube tightly, using a polyethylene stopper, and shake vigorously for 2 min, occasionally relieving the pressure from the sulfur dioxide by loosening the stoppers.
Centrifuge the tubes, withdraw most of the upper layer by means of a syringe, avoiding withdrawal of any of the lower, aqueous phase, and transfer the toluene extracts to a tared evaporating dish. Repeat the extraction twice with additional 15-mL portions of toluene, and evaporate the combined extracts on a steam bath to dryness. Dry the residue at a pressure not exceeding 5 mm of mercury at 60° for 2 h.
Cool, and weigh.
Acceptance criteria: NMT 2.0% (USP 1-Dec-2021)
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at 25°, excursions permitted between 15° and 30°.
Change to read:
USP Reference Standards 〈11〉
USP Ethacrynic Acid RS
USP Ethacrynic Acid Related Compound A RS
(4-Butyryl-2,3-dichlorophenoxy)acetic acid.


