Ethacrynate Sodium for Injection
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
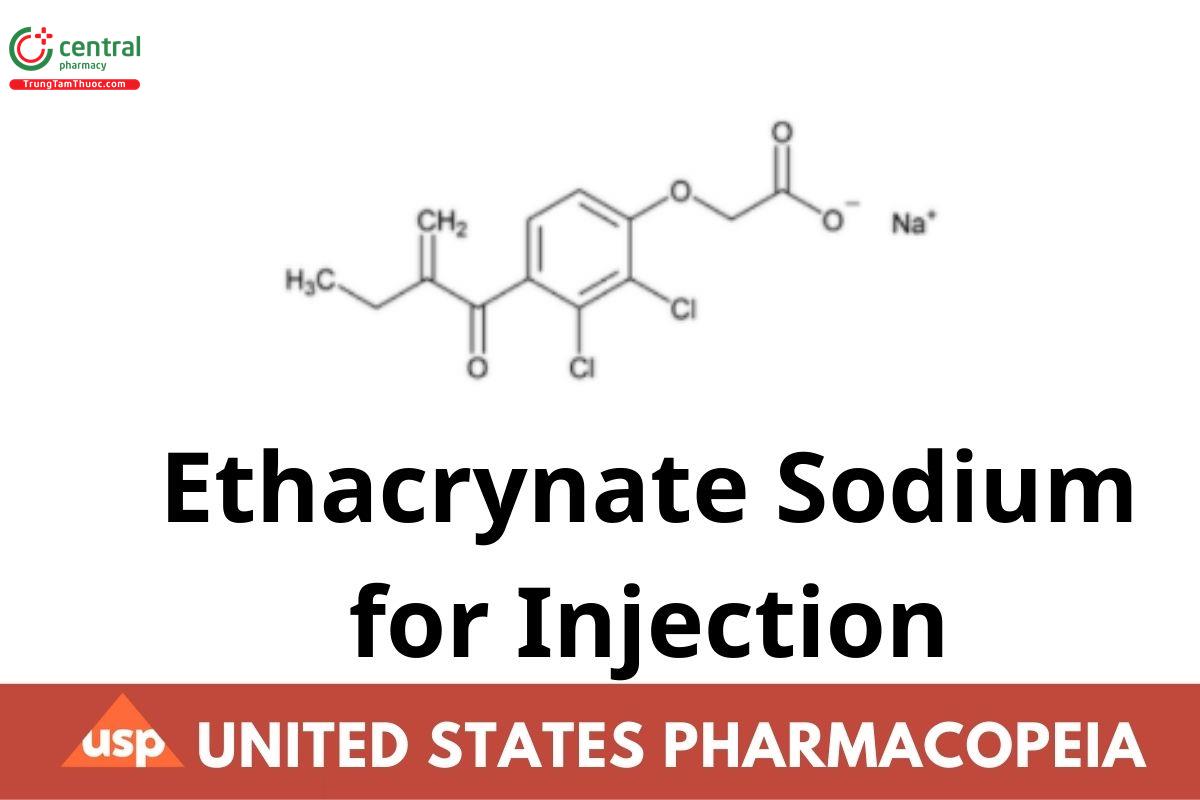
Ethacrynate Sodium for Injection is a sterile, freeze-dried powder prepared by the neutralization of Ethacrynic Acid with the aid of Sodium Hydroxide. It contains an amount of ethacrynate sodium equivalent to NLT 90.0% and NMT 110.0% of the labeled amount of ethacrynic acid (C13H12Cl2O4).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Diluent: Acidified methanol prepared by adding 9 mL of hydrochloric acid to 100 mL of methanol
Sample solution: 50 μg/mL in Diluent
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Buffer: Mix 10 mL of triethylamine and about 900 mL of water in a 1-L volumetric flask. Adjust with phosphoric acid to a pH of 6.8 ± 0.1, and dilute with water to volume.
Diluent: Acetonitrile and water (20:30)
Mobile phase: Acetonitrile and Buffer (400:600)
Standard solution: 0.5 mg/mL of USP Ethacrynic Acid RS in Diluent
Sample stock solution: Nominally equivalent to 2.5 mg/mL of ethacrynic acid from a suitable number of containers of Ethacrynate Sodium for Injection prepared as follows. Combine the contents of a suitable number of containers of Ethacrynate Sodium for Injection, equivalent to about 500 mg of ethacrynic acid, in a 200-mL volumetric flask. Add 5 mL of Diluent to each container, mix to dissolve the contents, and combine the resulting solutions in the volumetric flask taken. Rinse each container with two additional 5-mL portions of Diluent, add the rinsings to the solution in the volumetric flask, and dilute with Diluent to volume.
Sample solution: 0.5 mg/mL of ethacrynic acid in Diluent from Sample stock solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm × 30-cm; packing L1
Flow rate: 1 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Column efficiency: NLT 1200 theoretical plates
Tailing factor: NMT 2
Capacity factor, k′: NLT 0.8
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of ethacrynic acid (C13H12Cl2O4) in the portion of Ethacrynate Sodium for Injection taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Ethacrynic Acid RS in the Standard solution (mg/mL)
CU = nominal concentration of ethacrynic acid in the Sample solution (mg/mL)
Acceptance criteria: 90.0%–110.0%
4 PERFORMANCE TESTS
Uniformity of Dosage Units 〈905〉: Meets the requirements
5 SPECIFIC TESTS
pH 〈791〉
Sample solution: Equivalent to 1 mg/mL of ethacrynic acid in Sterile Water for Injection
Acceptance criteria: 5.0–7.0
Sterility Tests 〈71〉: Meets the requirements
Bacterial Endotoxins Test 〈85〉: NMT 5.0 USP Endotoxin Units/mg of ethacrynate sodium
Constituted Solution: At the time of use, it meets the requirements in Injections and Implanted Drug Products 〈1〉, Specific Tests, Completeness and clarity of solutions.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Packaging and Storage Requirements 〈659〉, Injection Packaging, Packaging for constitution .
Labeling: Label it to indicate that it was prepared by freeze-drying, having been filled into its container in the form of a true solution.
USP Reference Standards 〈11〉
USP Ethacrynic Acid RS


