Estradiol Vaginal Inserts
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Estradiol Vaginal Inserts contain NLT 90% and NMT 107% of the labeled amount of estradiol (C18H24O2).
2 IDENTIFICATION
A. Thin-Layer Chromatographic Identification Test 〈201〉
[Note—When two different concentrations are given for a solution, the lower concentration is for Inserts labeled to contain 0.01 mg of estradiol, and the high value is for Inserts labeled to contain 0.025 mg of estradiol.]
Standard solution: 2.5 mg/mL of USP Estradiol RS in absolute alcohol
Sample solution: Place a number of Inserts, equivalent to 0.38 or 0.95 mg of estradiol, into a vessel. Add 50 mL of isopropyl alcohol, and allow to disintegrate by stirring overnight. Centrifuge the suspension. Evaporate an aliquot of 40 mL of the supernatant to dryness, and dissolve the residue in 3 mL of isopropyl alcohol. Evaporate to dryness, reconstitute with 300 μL of absolute alcohol to obtain a solution containing 1.0 or 2.5 mg/mL of estradiol, and centrifuge.
Adsorbent: Use a suitable, high-performance thin-layer chromatographic plate.
Application volume: NLT 5 μL (equivalent to 10 μg of estradiol)
Developing solvent system: Chloroform and acetone (9:1)
Analysis: Proceed as directed in the chapter, using the Developing solvent system described above. Develop the chromatogram over a path of a minimum of 8 cm, and allow the plate to air-dry. Remove the plate, mark the solvent front, and allow solvent evaporation as described in the chapter. Heat at 100° for about 15 min. Allow the plate to cool, and then immerse it in a mixture of absolute alcohol and concentrated sulfuric acid (95:5). Remove it immediately, place the plate on absorbing paper, and allow it to air-dry. Heat the plate at 100° until the sulfuric acid has evaporated. Examine under UV light at λ = 365 nm.
Acceptance criteria: The principal spot obtained from the Sample solution has the same color and R value as that from the Standard solution.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
[Note—When two different concentrations are given for a solution, the lower concentration is for Inserts labeled to contain 0.01 mg of estradiol, and the high value is for Inserts labeled to contain 0.025 mg of estradiol.]
Mobile phase: Acetonitrile and water (11:9)
Diluent: Absolute alcohol and water (1:1)
Estrone standard stock solution: 0.1 mg/mL of USP Estrone RS in absolute alcohol
Estradiol standard stock solution: 0.25 mg/mL of USP Estradiol RS in absolute alcohol
System suitability solution: 0.6 and 2.0 μg/mL of USP Estrone RS and USP Estradiol RS in Diluent from Estrone standard stock solution and
Estradiol standard stock solution, respectively
Standard solution: 1.0 or 2.5 μg/mL of USP Estradiol RS in Diluent from Estradiol standard stock solution
Sample solution: 1.0 or 2.5 μg/mL of estradiol prepared using 10 Inserts in Diluent. Stir the mixture overnight with a magnetic stirrer, shake thoroughly, and centrifuge if necessary.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 3.9-mm × 30-cm; 4-μm packing L1
Flow rate: 1 mL/min
Injection size: 20 μL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 2.0 between estradiol and estrone
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of estradiol (C18H24O2) in the portion of Inserts taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Estradiol RS in the Standard solution (mg/mL)
CU = nominal concentration of estradiol in the Sample solution (mg/mL)
Acceptance criteria: 90%–107%
4 PERFORMANCE TESTS
Dissolution 〈711〉
Medium: Phosphate buffer pH 4.75 ± 0.05 (100 g of potassium dihydrogen phosphate in 10 L of water, adjusted with 1 N sodium hydroxide to a pH of 4.75 ± 0.05); 500 mL
Apparatus 1: 40 rpm
Time: 3, 5, and 10 h
Mobile phase: Methanol, acetonitrile, and water (27.5:27.5:45)
Standard stock solution: 0.1 mg/mL of USP Estradiol RS in absolute alcohol
Standard solutions: Quantitatively dilute with water the Standard stock solution to obtain solutions with final concentrations equal to approximately 20%, 60%, and 160% of the expected concentration of estradiol in the Medium for Inserts containing 0.025 mg, assuming complete dissolution, and approximately 20%, 50%, 150%, and 400% of the expected concentration of estradiol in the Medium for Inserts containing 0.01 mg of estradiol, assuming complete dissolution.
Sample solutions: Use the solution under test, unfiltered.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Fluorescence
Excitation wavelength: 230 nm
Emission wavelength: 310 nm
Column: 4.6-mm × 15-cm; 3.5-μm packing L1; or 4.6-mm × 7.5-cm; 5.0-μm packing L1
Flow rate: 1 mL/min
Injection size: 200 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.8
Relative standard deviation: NMT 2%
Analysis
Samples: Standard solution and Sample solution
Record the chromatograms, and measure the responses for the estradiol peak. Construct a calibration curve by plotting the peak response versus concentration of the Standard solutions. Determine the amount of estradiol (C18H24O2) in the Sample solutions from a linear regression analysis of the calibration curve.
Calculate the amount of estradiol (C18H24O2) dissolved:
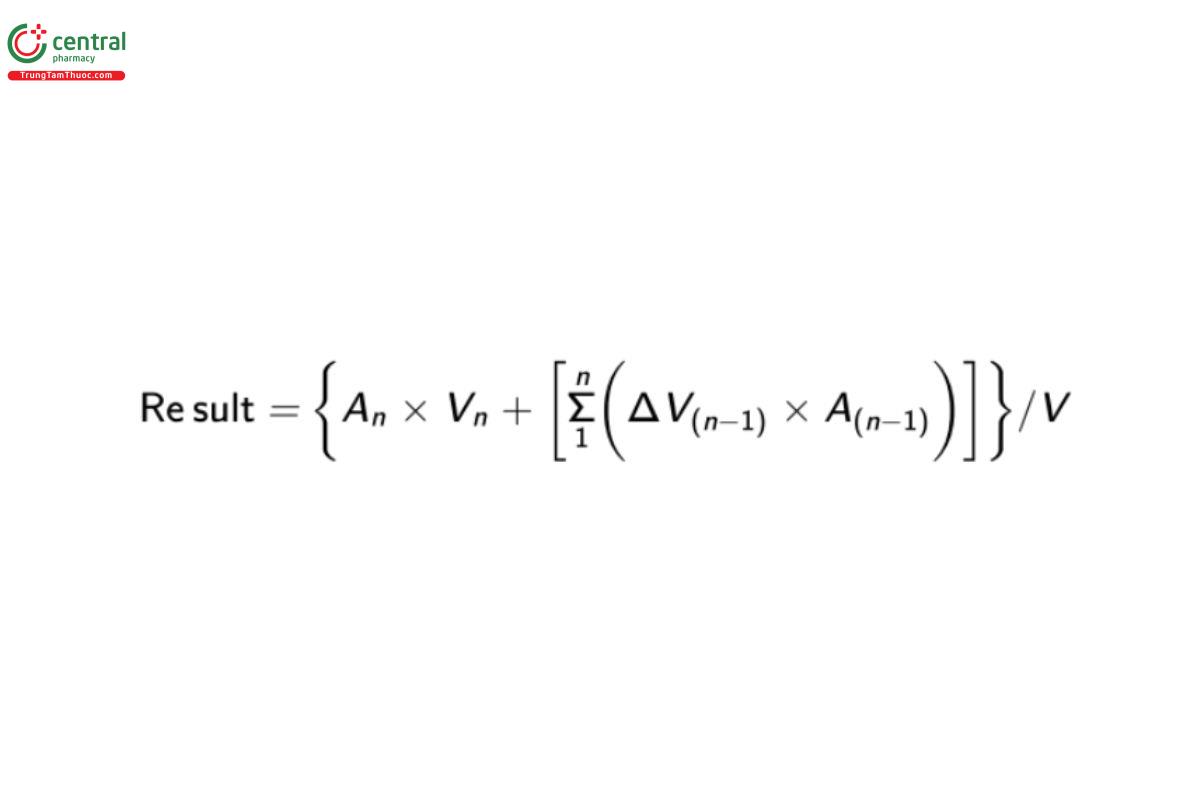
An = percentage of estradiol, at the sample point n (e.g., A at the second sampling point)
Vn = volume of Medium in the vessel before the sample is taken (mL)
ΔV(n-1) = volume of sample taken at the sampling point (n – 1)
A(n-1) = amount of estradiol (uncorrected) at the sample point (n – 1)
V = volume of the medium, 500 mL
Tolerances: See Table 1.
Table 1
Time (h) | Amount Dissolved (%) |
| 3 | 25-50 |
| 5 | 40-80 |
| 10 | NLT 80 |
The percentage of the labeled amount of estradiol dissolved at the specified times conforms to Acceptance Table 2 in 〈711〉.
5 IMPURITIES
Organic Impurities
[Note—When two different concentrations are given for a solution, the lower concentration is for Inserts labeled to contain 0.01 mg of estradiol, and the high value is for Inserts labeled to contain 0.025 mg of estradiol.]
Solution A: Acetonitrile
Solution B: Water
Mobile phase: See Table 2.
Table 2
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 16 | 84 |
| 35 | 68 | 32 |
[Note—Before the next injection, run the system at the initial condition until equilibration is achieved.]
System suitability solution: 100 μg/mL of USP Estradiol RS, 0.5 μg/mL of USP Estradiol Related Compound B RS, and 0.5 μg/mL of USP
Estradiol Related Compound C RS in absolute alcohol
Sample solution: Place a number of Inserts into a measured volume of absolute alcohol to obtain a solution having an estradiol concentration of 2.4 or 6.0 μg/mL. Stir for a minimum of 16 h, shake thoroughly, and centrifuge if necessary. Evaporate 10.0 mL of the supernatant to dryness. Dissolve the residue in 1.0 mL of water and add 7.0 mL of a mixture of toluene and acetone (5:2), mix on a whirl mixer, allow to stand for 1 h, and evaporate 5 mL of the organic phase to dryness. The residue is reconstituted in 450 μL of absolute alcohol to obtain a solution containing 38 or 95 μg/mL of estradiol. Centrifuge, and use the supernatant as the Sample solution.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Flow rate: 1 mL/min
Injection size: 25 μL
System suitability
Sample: System suitability solution
[Note—The relative retention times for estradiol related compound B and estradiol are about 0.96 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between estradiol related compound B and estradiol
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the Inserts taken:
Result = (rU/rS) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of estradiol from the Sample solution
Acceptance criteria: See Tables 3 and 4.
Table 3. For Inserts labeled to contain 0.025 mg of estradiol
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
Estradiol related compound C (6-ketoestradiol)a | 0.71 | 2.4 |
Estradiol related compound B (6-dehydroestradiol)b | 0.96 | 1.4 |
| Estradiol | 1.0 | - |
Any other individual impurity | - | 0.8 |
| Total impurities | - | 4.4 |
a 1,3,5(10)-Estratrien-3,17β-diol-6 one.
b 1,3,5(10),6-Estratetraen-3,17β-diol.
Table 4. For Inserts labeled to contain 0.01 mg of estradiol
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
Estradiol related compound C (6-ketoestradiol)a | 0.71 | 1.5 |
Estradiol related compound B (6-dehydroestradiol)b | 0.96 | - |
| Estradiol | 1.0 | - |
Any other individual impurity | - | 1.3 |
| Total impurities | - | 4.0 |
a 1,3,5(10)-Estratrien-3,17β-diol-6-one.
b 1,3,5(10),6-Estratetraen-3,17β-diol.
6 SPECIFIC TESTS
Microbial Enumeration Tests 〈61〉andTests for Specified Microorganisms 〈62〉: The total aerobic microbial count does not exceed 100 cfu/g, and the total combined molds and yeasts count does not exceed 10 cfu/g. Inserts meet the requirements of the tests for absence of Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans.
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in a tight container, and store at controlled room temperature. Do not refrigerate.
USP Reference Standards 〈11〉
USP Estradiol RS
USP Estradiol Related Compound B RS
6-Dehydroestradiol.
USP Estradiol Related Compound C RS
1,3,5(10)-Estratrien-3,17β-diol-6 one.
C18H22O3
USP Estrone RS


