Estradiol Benzoate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
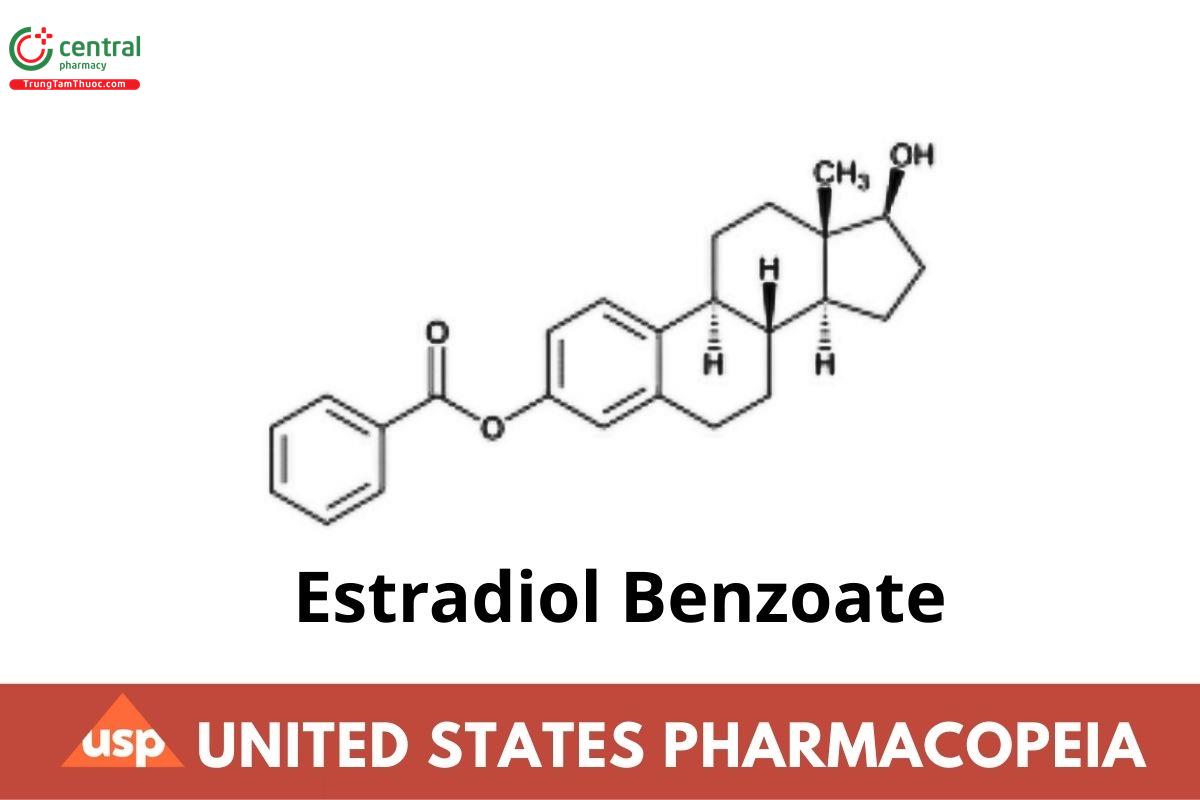
Estra-1,3,5(10)-triene-3,17-diol, (17β)-, 3-benzoate;
Estradiol 3-benzoate CAS RN®: 50-50-0; UNII: 1S4CJB5ZGN.
1 DEFINITION
Estradiol Benzoate contains NLT 97.0% and NMT 103.0% of estradiol benzoate (C25H28O3), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A or 197K [Note-If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in acetone, evaporate to dryness, and record new spectra using the residues.]
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Mobile phase: Acetonitrile and water (7:3)
System suitability solution: Transfer 20.0 mg each of USP Estradiol Benzoate RS and estradiol 17-acetate to a 100-mL volumetric flask. Add 70 ml. of acetonitrile, and sonicate until dissolved. Add 25 mL of water, mix well, and allow to equilibrate to ambient temperature. Dilute with water to volume.
Standard solution: Transfer 20.0 mg of USP Estradiol Benzoate RS to a 100-ml, volumetric flask. Add 70 mL of acetonitrile, and sonicate until dissolved. Add 25 mL of water, mix well, and allow to equilibrate to ambient temperature. Dilute with water to volume.
Sample solution: Transfer 20.0 mg of Estradiol Benzoate to a 100-ml volumetric flask. Add 70 ml of acetonitrile, and sonicate until dissolved. Add 25 ml. of water, mix well, and allow to equilibrate to ambient temperature. Dilute with water to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 230 nm
Columns
Guard: 4.6-mm × 4.5-cm; packing L1
Analytical: 4.6-mm × 25-cm; 5-μm packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for estradiol 17-acetate and estradiol benzoate are about 0.5 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 6.0 between estradiol 17-acetate and estradiol benzoate, System suitability solution
Tailing factor: NMT 2.0, System suitability solution
Relative standard deviation: NMT 1.5%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of estradiol benzoate (C25H28O3) in the portion of Estradiol Benzoate taken:
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Estradiol Benzoate RS in the Standard solution (mg/mL)
CU = concentration of Estradiol Benzoate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%-103.0% on the dried basis
4 IMPURITIES
RESIDUE ON IGNITION (281)
Sample: 250 mg
Acceptance criteria: NMT 0.2%
LIMIT OF METHANOL AND DICHLOROMETHANE: Proceed as directed in Residual Solvents (467),
Acceptance criteria: The sum of methanol and methylene chloride is NMT 0.20%,
ORGANIC IMPURITIES
Diluent: Methylene chloride and alcohol (2:1)
Standard solution: 5 mg/mL of USP Estradiol Benzoate RS in Diluent
Sample solution A: 5 mg/ml of Estradiol Benzoate in Diluent
Sample solution B: 0.1 mg/mL of Estradiol Benzoate in Diluent from Sample solution A
Chromatographic system
(See Chromatography (621), General Procedures. Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Developing solvent system: Toluene and ethyl acetate (70:30)
Spray reagent: 50 mg/mL of ammonium molybdate in 10% sulfuric acid
Analysis
Samples: Standard solution, Sample solution A, and Sample solution B
To the plate apply 20-µL aliquots of the Standard solution and Sample solution A and 20-, 15, 10-, 5-, and 2-ul, aliquots of Sample solution
B. The volumetric series of Sample solution B represents 2.0%, 1.5%, 1.0%, 0.5%, and 0.2% of the concentration of Estradiol Benzoate within the Sample solution A spot.
Allow the spots to dry, and develop the chromatogram until the solvent front has moved about three-fourths of the length of the plate. Spray the plate thoroughly with Spray reagent, and dry. Heat the plate in a drying oven for 10 min at about 115".
Calculate the relative retardation factor (relative to estradiol benzoate) of all spots within the lanes for Sample solution A and Sample solution B.
Determine the percentages of each impurity by comparing the intensity of the impurity spots within Sample solution A to those of the main spots obtained from the series of Sample solution B, ignoring any impurity peak less intense than the main spots found in the Sample solution B lane containing 0.2% of the amount of estradiol benzoate of Sample solution A.
Acceptance criteria: See Table 1.
| Name | Relative Retardation Factor | Acceptance Criteria, NMT (%) |
| Estradiola | 0.84 | 1.0 |
| Estradiol benzoate | 1.00 | - |
| 17a-Estradiol benzoateb | 1.15 | 1.0 |
| Estronec | 1.21 | 1.0 |
| Any individual unspecified impurity | - | 1.0 |
| Total impurities | - | 2.0 |
a Estra-1,3,5(10)-triene-3,17b-diol.
b Estra-1,3,5(10)-triene-3,17a-diol 3-benzoate.
c Estra-1,3,5(10)-triene-17-one, 3-hydroxy.
5 SPECIFIC TESTS
OPTICAL ROTATION (781S), Procedures, Specific Rotation
Sample solution: 10 mg/mL, previously dried in dioxane
Acceptance criteria: +57.0° to +63.0°
LOSS ON DRYING (731),
Analysis: Dry at 100° -105° for 3 h.
Acceptance criteria: NMT 0.5%
PARTICLE SIZE DISTRIBUTION ESTIMATION BY ANALYTICAL SIEVING (786)
Suspension fluid: To a mixture of Glycerin and water (60:40, w/w) add a sufficient quantity of polysorbate 20 to obtain a solution having a concentration of 125 µl of polysorbate 20 per 100 g of solution.
Sample suspension: Saturate the Suspension fluid by adding 100 mg of fine Estradiol Benzoate per 100 g of Suspension fluid, and sonicate for 10 min. Pass the resulting suspension through a nylon filter of 0.45-um pore size. To the filtrate, add 50 mg of Estradiol Benzoate per mL of the filtered, saturated Suspension fluid, and mix on a vortex mixer until dispersed (about 1 min).
Analysis: Using a suitable multi-wavelength particle size analyzer, determine the particle size distribution within the Sample suspension, analyzing the results in the range of 5-600 µm.
Acceptance criteria: NMT 50% of the particles are less than 30 µm, and NLT 90% of the particles are less than 450 µm. The mean diameter of fine grade Estradiol Benzoate is NMT 100 µm, and the mean diameter of coarse grade Estradiol Benzoate is 100-200 µm.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
LABELING: Label it to indicate that it is for veterinary use only. Label it to indicate whether it is coarse grade or fine grade.


