Escitalopram Oxalate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
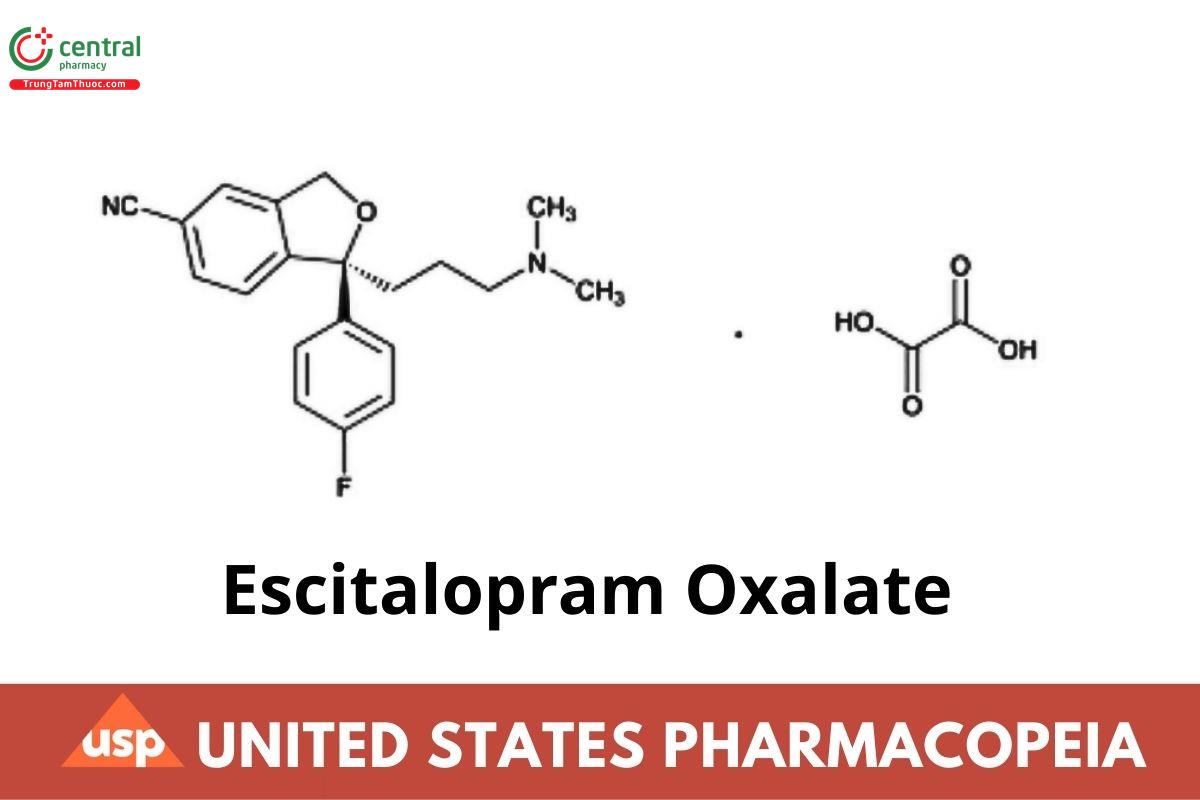
S-(+)-5-Isobenzofurancarbonitrile, 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-, oxalate;
S-(+)-1-[3-(Dimethylamino)propyl]-1-(p-fluorophenyl)-5-phthalancarbonitrile oxalate CAS RN®: 219861-08-2; UNII: 5U85DBW7LO.
1 DEFINITION
Escitalopram Oxalate contains NLT 98.0% and NMT 102.0% of Escitalopram oxalate (C20H21FN2O · C2H2O4), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Buffer: 3.4 g/L of monobasic potassium phosphate in water. Adjust with phosphoric acid or sodium hydroxide solution to a pH of 3.0 before final dilution.
Solution A: Acetonitrile and Buffer (10:90)
Solution B: Acetonitrile and Buffer (65:35)
Mobile phase: See Table 1
| Time (min) | Solution A (%) | Solution B (%) | Flow Rate (mL/min) |
| 0 | 95 | 5 | 1 |
| 35 | 65 | 35 | 1 |
| 45 | 0 | 100 | 1 |
| 45.1 | 0 | 100 | 2 |
| 60 | 0 | 100 | 2 |
| 60.1 | 95 | 5 | 1 |
| 68 | 95 | 5 | 1 |
[Note-The gradient was established on an HPLC system with a dwell volume of approximately 1.6 mL]
System suitability solution: 2 µg/mL each of USP Escitalopram Oxalate RS and USP Citalopram Related Compound D. RS in Solution A
Standard solution: 0.5 mg/mL of USP Escitalopram Oxalate RS in Solution A
Sample solution: 0.5 mg/ml of Escitalopram Oxalate in Solution A
Chromatographic system
(See Chromatography (621), System Surtability)
Mode: LC
Detector: UV 237 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Column temperature: 45°
Flow rate: See Table 1.
Injection volume: 20 μL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.5 between escitalopram and citalopram related compound D, System suitability solution
Tailing factor: 0.8-3, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of escitalopram oxalate (C20H21FN2O · C2H2O4) in the portion of Escitalopram Oxalate taken
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%
ENANTIOMERIC PURITY
Buffer: Dissolve 6.8 g of monobasic potassium phosphate in 250 ml of water, add 150 mL of 0.2 N sodium hydroxide, adjust with phosphoric
acid or sodium hydroxide solution to a pH of 7.0, and dilute with water to 1 L
Mobile phase: Acetonitrile and Buffer (15:85)
System suitability solution: 125 µg/ml. each of USP R-Citalopram Oxalate RS and USP Escitalopram Oxalate RS in Mobile phase
Sample solution: 125 µg/ml. of Escitalopram Oxalate in Mobile phase
Chromatographic system
(See Chromatography (621). System Suitability)
Mode: LC
Detector: UV 240 nm
Column: 4.6-mm × 15-cm; 5-μm packing L57
Column temperature: 30°
Flow rate: 0.6 mL/min
Injection volume: 15 μl
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 1.3 between R-citalopram and escitalopram
Tailing factor: 0.8-2.5 for escitalopram
Analysis
Sample: Sample solution
Calculate the percentage of R-citalopram oxalate in the portion of Escitalopram Oxalate taken:
Result = (ru/rT) x 100
ru = peak response of R-citalopram from the Sample solution
rT = sum of peak responses of R-citalopram and escitalopram from the Sample solution
Acceptance criteria: NMT 3.0%
ORGANIC IMPURITIES
Buffer, Solution A, Solution B, Mobile phase, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability solution A: 2 µg/ml. each of USP Escitalopram Oxalate RS and USP Citalopram Related Compound D RS in Solution A
System suitability solution 8: 0.5 mg/ml of USP Escitalopram Oxalate RS in Solution A
System suitability
Samples: System suitability solution A and System suitability solution B
Suitability requirements
Resolution: NLT 1.5 between escitalopram and citalopram related compound D, System suitability solution A
Tailing factor: 0.8-3, System suitability solution B
Relative standard deviation: NMT 2.0%, System suitability solution B
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Escitalopram Oxalate taken.
Result = (ru/rT) x (1/F) x100
ru = peak response of each impurity from the Sample solution
rT = peak response of escitalopram from the Sample solution
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Oxalic acida | 0.075 | - | - |
| 5-Dimethylamino butyryl citalopramb | 0.40 | 0.34 | 0.2 |
| Citalopram related compound Ac | 0.50 | 0.79 | 0.1 |
| Citalopram related compound B (3-hydroxycitalopram)d | 0.74 | 1.0 | 0.1 |
| Citalopram related compound C (3-oxocitalopram)e | 0.90 | 0.79 | 0.1 |
| Citalopram related compound D (desmethyl citalopram) | 0.97 | 1.0 | 0.1 |
| Escitalopram | 1.0 | - | - |
| Citalopram related compound E (citalopram N-oxide)f | 1.1 | 1.0 | 0.1 |
| Any other individual unspecified impurity | - | 1.0 | 0.1 |
| Total impurities | - | - | 0.5 |
a Included for identification only. This peak is due to the oxalate counterion and hence is not an impurity.
b 1-(3-Dimethylaminopropyl)-1-(4′-fluorophenyl)-5-(4-dimethylaminobutyryl)-1,3-dihydroisobenzofuran.
c 1-(3-Dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxamide.
d 1-(3-Dimethylaminopropyl)-1-(4-fluorophenyl)-3-hydroxy-1,3-dihydroisobenzofuran-5-carbonitrile.
e 3-(3-Dimethylaminopropyl)-3-(4-fluorophenyl)-6-cyano-1(3H)-isobenzofuranone.
f 1-(3-Dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile-N-oxide.
5 SPECIFIC TESTS
Water Determination, Method Ia (921): NMT 1.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.


