Erythromycin Ethylsuccinate Tablets
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Erythromycin Ethylsuccinate Tablets contain the equivalent of NLT 90.0% and NMT 120.0% of the labeled amount of erythromycin (C37H67NO13).
2 IDENTIFICATION
A. THIN-LAYER CHROMATOGRAPHY
Standard solution: 3 mg/mL of USP Erythromycin Ethylsuccinate RS in methanol
Sample solution: Nominally 2.5 mg/mL of erythromycin from powdered Tablets in methanol. Shake this mixture by mechanical means for about 30 min. Centrifuge a portion of this mixture, and use the clear supernatant as the Sample solution.
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL
Developing solvent system: Methanol and chloroform (85:15)
Spray reagent: Dehydrated alcohol, p-methoxybenzaldehyde, and sulfuric acid (90:5:5)
Analysis
Samples: Standard solution and Sample solution
Place the plate in an unlined chromatographic chamber, and develop in the Developing solvent system until the solvent front has moved about 9 cm. Remove the plate from the chamber, mark the solvent front, and allow the solvent to evaporate. Spray the plate with the Spray reagent. Heat it at 100° for 10 min, and examine the chromatograms. The erythromycin and succinic acid moieties appear as black-to-purple spots.
Acceptance criteria: The R, values of the principal spots from the Sample solution correspond to those from the Standard solution.
3 ASSAY
PROCEDURE
(See Antibiotics-Microbial Assays (81).)
Test dilution: Blend NLT 4 Tablets for 4 + 1 min in a high-speed glass blender jar with a sufficient volume of methanol to give a stock solution containing nominally NMT 5 mg/mL of erythromycin. Dilute this stock solution quantitatively with Buffer B.3 to obtain a Test dilution having a concentration assumed to be equal to the median dose level of the standard. dose level
Analysis: Proceed as directed in the chapter.
Acceptance criteria: 90.0%-120.0%
4 PERFORMANCE TESTS
DISSOLUTION (711)
4.1 FOR NONCHEWABLE TABLETS
Medium: 0.01 N hydrochloric acid; 900 ml.
Apparatus 2: 50 rpm
Time: 45 min
Solution A: 25 mg/mL of ferric chloride
Solution B: Slowly, and with constant mechanical stirring, add 325 mL of sulfuric acid to 173 mL of cold water. Allow the solution to cool, add 2 mL of Solution A and 1 g of p-dimethylaminobenzaldehyde, and stir to dissolve. Store in a low-actinic flask. Prepare Solution B on the day of use.
Standard solution: 0.44 mg/mL of USP Erythromycin RS in Medium. Sonicate as necessary to dissolve. Use the Standard solution within 5.5 h.
Sample solution: Pass a portion of the solution under test through a filter having a pore size of 0.5 µm or less, discarding the first 5 mL of the filtrate.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857).)
Mode: Vis
Analytical wavelength: 480 nm
Blank: Medium
Analysis
Samples: Standard solution and Sample solution
To three separate 50-ml glass-stoppered conical flasks add 2.0 mL of Standard solution, 2.0 mL of Sample solution, and 2.0 mL of the Blank. Place the flasks in an ice bath for about 15 min. At precise 1-min intervals add 10.0 mL of Solution B to the Standard solution, the Sample solution, and the Blank, in turn. Immediately after adding Solution B, remove each flask from the ice bath, insert the stopper, mix, and allow to stand at room temperature for exactly 30 min. Sequentially determine the absorbance at 480 nm of the Standard solution and the Sample solution at precise 1-min intervals using the Blank to set the spectrophotometer to zero.
Calculate the percentage of the labeled amount of erythromycin (C37H67NO13) dissolved:
Result = (Au /As ) × Cs × V × P × F × (1/L) × 100
Au = absorbance of the Sample solution
As = absorbance of the Standard solution
Cs = concentration of USP Erythromycin RS in the Standard solution (mg/mL)
V = volume of Medium, 900 mL
P = potency of erythromycin in USP Erythromycin RS (µg/mg)
F = conversion factor, 0.001 mg/µg
L = label claim (mg/Tablet)
Acceptance criteria: NLT 75% (Q) of the labeled amount of erythromycin (C37H67NO13) is dissolved.
4.2 FOR TABLETS LABELED AS CHEWABLE
Medium: 0.1 M acetate buffer, pH 5.0; 900 mL
Apparatus 2: 75 rpm
Time: 60 min
Buffer A: 33.25 g/L of sodium acetate in water. Adjust with glacial acetic acid to a pH of 4.8 ± 0.1 before diluting to final volume.
Buffer B: pH 7.6 phosphate buffer (see Reagents, Indicators, and Solutions-Solutions)
Standard stock solution: 0.5 mg/mL of USP Erythromycin RS in Buffer A
Standard solutions: Transfer 8.0, 4.0, and 1.0-mL volumes of the Standard stock solution to separate 100-mL volumetric flasks, add 6.0 mL of Buffer B and 6.0 ml of Medium to each flask, and dilute with Buffer A to volume. The Standard solutions have known concentrations of about 40, 20, and 5 µg/mL of USP Erythromycin RS, respectively.
Sample solution: Transfer 6.0 ml of the solution under test to a 50-mL volumetric flask, add 6.0 mL of Buffer B, and heat in boiling water for 30 min. Cool to room temperature, and dilute with Buffer A to volume.
Arsenomolybdate stock solution: Dissolve 100 g of ammonium molybdate in 1.7 L of water in a 2-L volumetric flask. Slowly add, with mixing, 84 ml. of sulfuric acid. Add 12 g of sodium arsenate dissolved in 100 mL of water. Dilute with water to volume. Store in an amber bottle for 24 h before using. This solution should not come in contact with rubber.
Arsenomolybdate solution: Water and Arsenomolybdate stock solution (2:1). Prepare freshly on the day of use.
4.5 M sulfuric acid: Add 1.5 L of water to a 2-L volumetric flask, and place the flask in an ice bath. Slowly add, with stirring, 300 mL of sulfuric acid. Allow the solution to cool, dilute with water to volume, and mix. At the time of use, add 0.5 g of sodium dodecyl sulfate to each L.
Apparatus: To determine the absorbance values, use an automated analyzer consisting of a liquid sampler, a proportioning pump; a manifold; and a spectrophotometer equipped with matched flow cells, suitable recording devices, and analysis capability at 660 nm. See Figure 1.
Figure 1
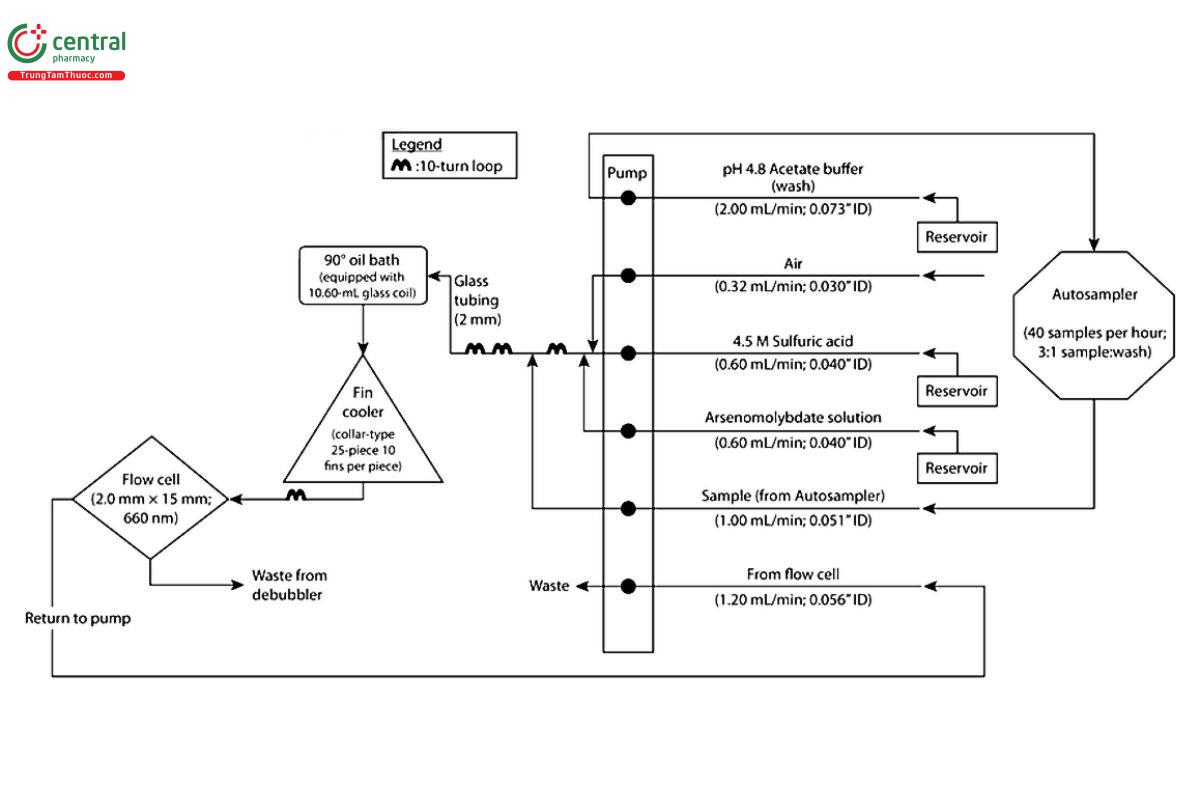
Analysis
Samples: Standard solutions and Sample solution
Adjust the system until a steady baseline is achieved. Start the sampler, and conduct determinations at a rate of about 40–60/h with a 3:1 (sample/wash) ratio. Adjust the analyzer ow rates, if necessary, to optimize system performance.
Calculate the percentage of the labeled amount of erythromycin (C37H67NO13) dissolved:
Result = (Au /As ) × Cs × V × P × F × (1/L) × 100
Au = response of the Sample solution
As = response of the Standard solution
Cs = concentration of USP Erythromycin RS in the Standard solution (mg/mL)
V = volume of Medium, 900 mL
P = potency of erythromycin in USP Erythromycin RS (µg/mg)
F = conversion factor, 0.001 mg/µg
L = label claim (mg/Tablet)
Acceptance criteria: NLT 75% (Q) of the labeled amount of erythromycin (C37H67NO13) is dissolved
UNIFORMITY OF DOSAGE UNITS (905): Meet the requirements
5 SPECIFIC TESTS
LOSS ON DRYING (731)
Analysis: Dry 100 mg in vacuum at a pressure not exceeding 5 mm of mercury at 60° for 3 h.
Acceptance criteria: NMT 4.0%; Chewable Tablets are exempt from this requirement.
WATER DETERMINATION, Method (921): NMT 5.0%; Chewable Tablets only
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, protect from light and moisture, and store at a temperature below 30°.
LABELING: Label the Chewable Tablets to indicate that they are to be chewed before swallowing.
USP REFERENCE STANDARDS (11)
USP Erythromycin RS
USP Erythromycin Ethylsuccinate RS


