Erythromycin Ethylsuccinate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
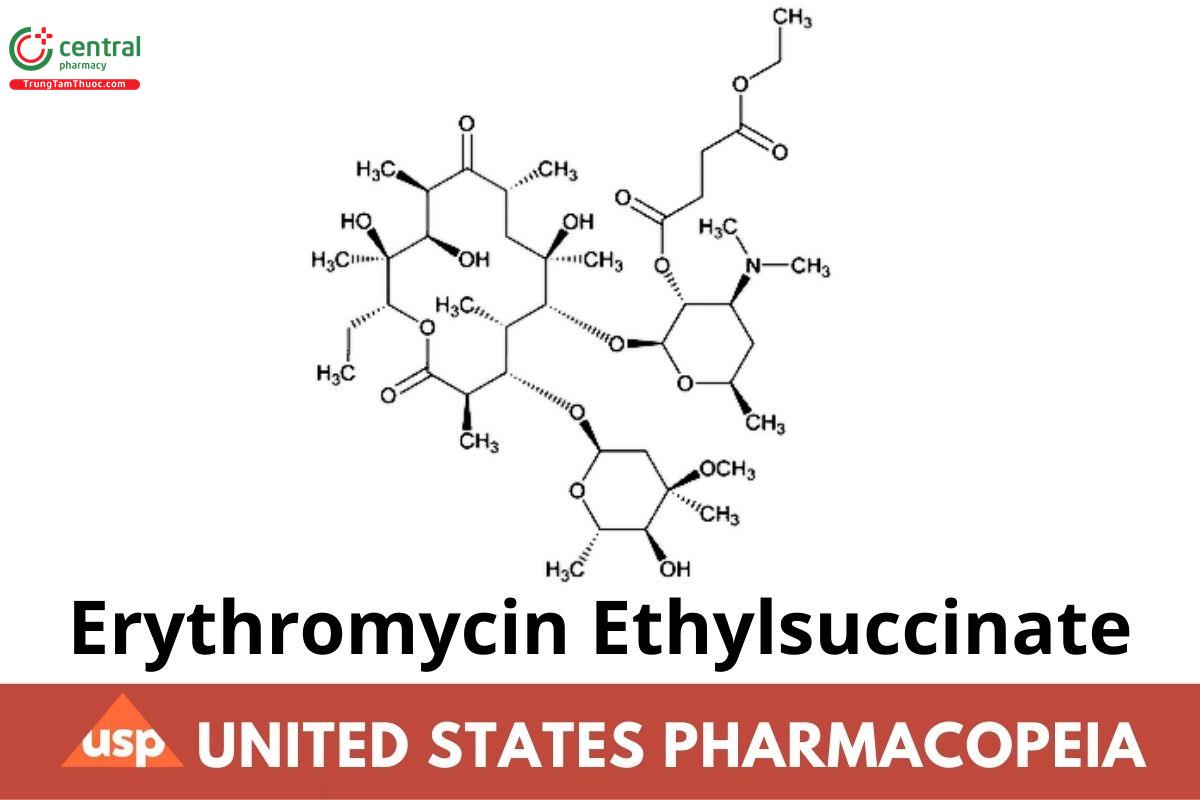
C43H75NO16 862.06
Erythromycin 2′-(ethyl butanedioate);
Erythromycin 2′-(ethyl succinate) CAS RN: 1264-62-6; UNII: 1014KSJ86F.
1 DEFINITION
Erythromycin Ethylsuccinate consists primarily of the 2'-ethylsuccinate ester of erythromycin A. The sum of the percentages of erythromycin A, erythromycin B, and erythromycin C is NLT 76.5%, calculated on the anhydrous basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 1975
Sample solution: 10 mg/mL in chloroform
Cell: 1.0 mm
Acceptance criteria: Meets the requirements
B. The retention times of the erythromycin A, erythromycin B, and erythromycin C peaks in the Sample solution correspond to those of Standard solution 1 and Standard solution 2, as obtained in the Assay.
3 ASSAY
PROCEDURE
Use solutions containing erythromycin promptly, or within 1 day if stored in a refrigerator.
Solution A: 20 mg/mL of dibasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 8.0.
Solution B: 35 mg/mL of dibasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 8.0.
Solution C: Adjust 20 mL of Solution A with phosphoric acid to a pH of 3.5.
Mobile phase: Acetonitrile, tertiary butyl alcohol, Solution B, and water (6:35:10:149)
Standard solution 1: 50 mg of USP Erythromycin RS in a 25-ml. volumetric flask. Add 12.5 mL of methanol, and swirl to dissolve. Dilute with Solution A to volume.
Standard solution 2: 5 mg each of USP Erythromycin B RS and USP Erythromycin C. RS, in a 50-ml, volumetric flask. Add 25.0 ml. of methanol, and swirl to dissolve. Add 2.5 mL of Standard solution 1, and dilute with Solution A to volume.
System suitability solution: 0.1 mg/mL of USP Erythromycin Related Compound N.RS in Standard solution 2
Sample solution: 115 mg of Erythromycin Ethylsuccinate in a 50-mL volumetric flask. Add 25.0 mL of methanol, and swirl to dissolve. Add about 20 mL of Solution A and allow to stand at room temperature for about 12 h to effect hydrolysis. Dilute with Solution A to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm x 25-cm; packing L21 (1000A)
Column temperature: 70°
Flow rate: 2 mL/min
Injection volume: 200 µL
Run time: NLT 5 times the retention time of erythromycin A
System suitability
Samples: Standard solution 1 and System suitability solution
The order of elution of the components is erythromycin related compound N, erythromycin C, erythromycin A, and erythromycin B.
Suitability requirements
Resolution: NLT 0.8 between erythromycin related compound N and erythromycin C; NLT 5.5 between erythromycin related compound N and erythromycin A, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution 1
Analysis
Samples: Standard solution 1, Standard solution 2, and Sample solution
Calculate the percentage of erythromycin A in the portion of Erythromycin Ethylsuccinate taken:
Result = (ru /rs ) × (Cs /Cu ) × P × 100
ru = peak response of erythromycin A from the Sample solution
rs = peak response of erythromycin A from Standard solution 1
Cs = concentration of USP Erythromycin RS in Standard solution 1 (mg/mL)
Cu = concentration of Erythromycin Ethylsuccinate in the Sample solution (mg/mL)
P = percentage of erythromycin A in USP Erythromycin RS
Calculate the percentage of erythromycin B and erythromycin C in the portion of Erythromycin Ethylsuccinate taken:
Result = (ru /rs ) × (Cs /Cu ) × P × 100
ru = peak response of the relevant analyte from the Sample solution
rs = peak response of the relevant analyte from Standard solution 2
Cs = concentration of the corresponding Reference Standard in Standard solution 2 (mg/mL)
Cu = concentration of Erythromycin Ethylsuccinate in the Sample solution (mg/mL)
P = potency of erythromycin B or erythromycin C in the corresponding Reference Standard (mg/mg)
Acceptance criteria: NLT 76.5% for the sum of the percentages of erythromycin A, erythromycin B, and erythromycin C, on the anhydrous basis. The percentage of erythromycin B is NMT 12.0%, and the percentage of erythromycin C is NMT 5.0%.
4 IMPURITIES
RESIDUE ON IGNITION (281)
Sample: After ignition at 550 ± 50o, the charred residue is moistened with 2 mL of nitric acid and 5 drops of sulfuric acid.
Acceptance criteria: NMT 1.0%
ORGANIC IMPURITIES
Solution B, Solution C, Mobile phase, Standard solution 1, Standard solution 2, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Peak identification solution: 10 mg of USP Erythromycin RS in 2 mL of methanol. Add 10 mL of Solution C, and allow to stand for about 30 min. Refrigerate this solution until used, and discard 8 h after preparation.
Analysis
Samples: Standard solution 2, Sample solution, and Peak identification solution
Identify the erythromycin A enol ether peak using the Peak identification solution. Begin peak integration of the Sample solution after the two peaks for succinates that elute just after the solvent front.
Calculate the percentage of the largest single impurity (the largest peak other than erythromycin A, erythromycin B, erythromycin C, erythromycin A enol ether, and erythromycin N-ethylsuccinate) in the portion of Erythromycin Ethylsuccinate taken:
Result = (ru /rs ) × (Cs /Cu ) × P × 100
ru = peak response of the largest single impurity (the largest peak other than erythromycin A, erythromycin B, erythromycin C, erythromycin A enol ether, and erythromycin N-ethylsuccinate) from the Sample solution
rs = peak response of erythromycin A from Standard solution 2
Cs = concentration of USP Erythromycin RS in Standard solution 2 (mg/mL)
Cu = concentration of Erythromycin Ethylsuccinate in the Sample solution (mg/mL)
P = percentage of erythromycin A in USP Erythromycin RS
Calculate the percentage of erythromycin A enol ether in the portion of Erythromycin Ethylsuccinate taken:
Result = (ru /rs ) × (Cs /Cu ) × P × (1/F) × 100
ru = peak response of erythromycin A enol ether from the Sample solution
rs = peak response of erythromycin A from Standard solution 2
Cs = concentration of USP Erythromycin RS in Standard solution 2 (mg/mL)
Cu = concentration of Erythromycin Ethylsuccinate in the Sample solution (mg/mL)
P = percentage of erythromycin A in USP Erythromycin RS
F = relative response factor (see Table 1)
Calculate the percentage of erythromycin N-ethylsuccinate in the portion of Erythromycin Ethylsuccinate taken:
Result = (ru /rs ) × (Cs /Cu ) × P × (1/F) × 100
ru = peak response of erythromycin N-ethylsuccinate from the Sample solution
rs = peak response of erythromycin A from Standard solution 2
Cs = concentration of USP Erythromycin RS in Standard solution 2 (mg/mL)
Cu = concentration of Erythromycin Ethylsuccinate in the Sample solution (mg/mL)
P = percentage of erythromycin A in USP Erythromycin RS
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1.
Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Erythromycin N-ethylsuccinatea | 1.3 | 7.4 | 3.0 |
| Erythromycin A enol etherb | 4.3-4.7 | 11 | 3.0 |
| Erythromycin A | 1.0 | - | - |
| Largest single impurityc | - | - | 3.0 |
a Ethyl 4-{[(2S,3R,4S,6R)-2-{[(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-14-ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6- dimethyltetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyl-2,10-dioxooxacyclotetradecan-6-yl]oxy}-3-hydroxy-6-methyltetrahydro-2H-pyran-4-yl](methyl)amino}-4-oxobutanoate.
b (2R,3R,4S,5R,8R,9S,10S,11R)-11-{[(2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-5-ethyl-3,4-dihydroxy9-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-2,4,8,10,12,14-hexamethyl-6,15-dioxabicyclo[10.2.1]pentadec-1(14)-en-7-one.
c The largest peak other than erythromycin A, erythromycin B, erythromycin C, erythromycin A enol ether, and erythromycin N-ethylsuccinate
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method I
Sample solution: Use 20 mL of methanol containing 10% of imidazole in place of methanol in the titration vessel.
Acceptance criteria: NMT 3.0%
CRYSTALLINITY (695): Meets the requirements except, where it is labeled as amorphous, most of the particles do not exhibit birefringence and extinction positions.
Change to read:
X-RAY POWDER DIFFRACTION (941) (CN 1-MAY-2022): Where labeled as being amorphous, its X-ray diffraction pattern performed at high sensitivity for angles of diffraction between 2° and 20° exhibits no reection, and between 7° and 10° exhibits a more intense hachured baseline, creating a halo for angles of diffraction between 2° and 20° exhibits no reection, and between 7° and 10° exhibits a more intense hachured baseline, creating a halo.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Erythromycin Ethylsuccinate that is noncrystalline is labeled to indicate that it is amorphous. Any preparation containing the amorphous form of Erythromycin Ethylsuccinate is so labeled.
USP Reference Standards 〈11〉
USP Erythromycin RS
USP Erythromycin B RS
12-Deoxyerythromycin; (3R,4S,5S,6R,7R,9R,11R,12S,13R,14R)-6-{[(2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-14-ethyl-7,12- dihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione.
C37H67NO12 717.94
USP Erythromycin C RS
3″-O-Demethylerythromycin;(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-{[(2R,4R,5S,6S)-4,5-Dihydroxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyloxacyclotetradecane2,10-dione.
C36H65NO13 719.91
USP Erythromycin Related Compound N RS
N-Demethylerythromycin A;(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-14-Ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-6-{[(2S,3R,4S,6R)-3-hydroxy-6-methyl-4-(methylamino)tetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13 hexamethyloxacyclotetradecane-2,10-dione.
C36H65NO13 719.91
USP Erythromycin Ethylsuccinate RS


