Erythromycin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
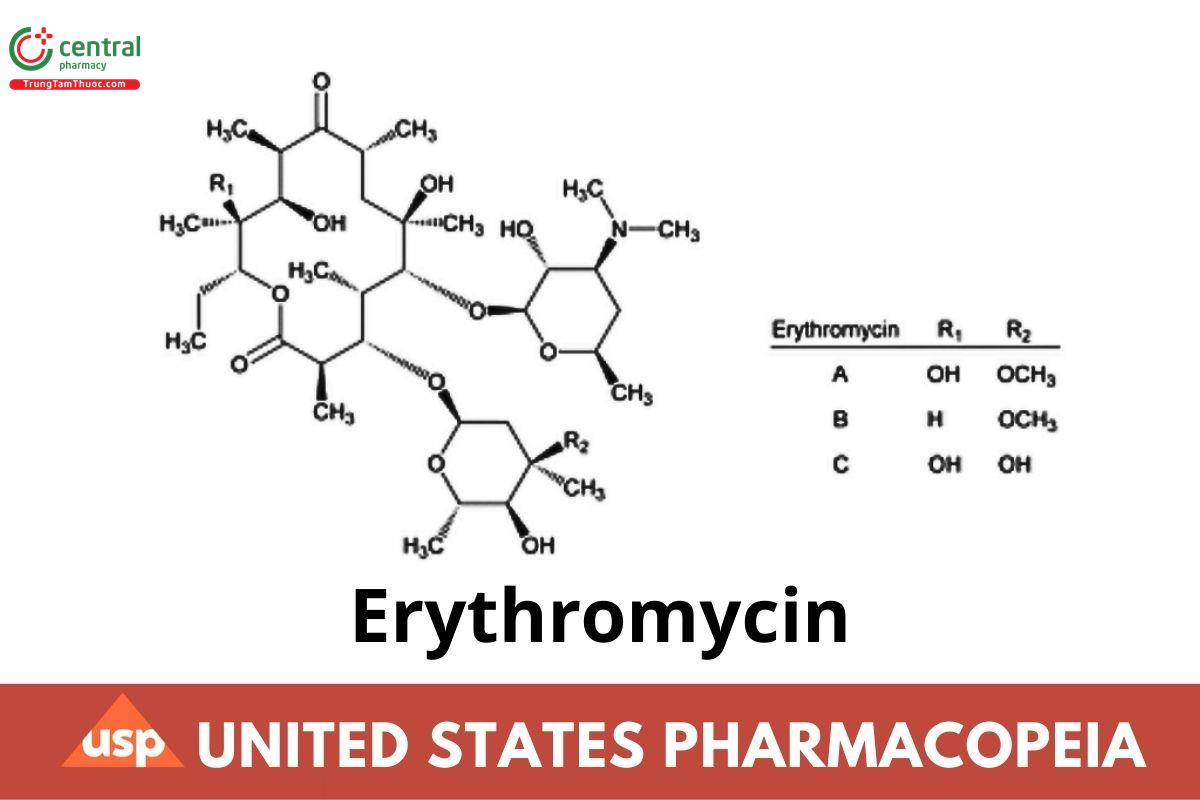
C37H67NO13
Erythromycin A
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[(3,4,6-trideoxy-3-dimethylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione;
C37H67NO12 717.94
Erythromycin B
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12-dihydroxy-3,5,7,9,11,13- hexamethyl-6-[(3,4,6-trideoxy-3-dimethylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione; 12-Deoxyerythromycin CAS RN®: 527-75-3.
C37H65NO12 719.91
Erythromycin C
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13- hexamethyl-6-[(3,4,6-trideoxy-3-dimethylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione; 3”-O-Demethylerythromycin CAS RN®: 1675-02-1.
Change to read:
1 DEFINITION
Erythromycin consists primarily of erythromycin A (C37H67NO13). The sum of the percentages of erythromycin A, erythromycin B, and erythromycin C is NLT 93.0% and NMT 102.0% calculated on the anhydrous basis.
IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 1975
Standard solution: 50 mg/mL of USP Erythromycin RS, previously dried at a pressure not exceeding 5 mm of mercury at 60 for 3 h, in chloroform
Sample solution: 50 mg/mL of Erythromycin, previously dried at a pressure not exceeding 5 mm of mercury at 60° for 3 h, in chloroform
Spectral range: 4000-2050 cm and 1980-400 cm
Acceptance criteria: Meets the requirements
B. The retention times of erythromycin A, erythromycin B, and erythromycin C in the Sample solution correspond to those of Standard solution T and Standard solution 2, as obtained in the Assay.
2 ASSAY
Change to read:
PROCEDURE
Prepare the erythromycin solutions immediately before use.
Diluted phosphoric acid: Dilute 7 mL of phosphoric acid with water to 100 ml.
Phosphate buffer solution pH 8.0: Dissolve 11.5 g of dibasic potassium phosphate in 900 ml of water. Adjust to a pH of 8.0 with Diluted phosphoric acid and dilute with water to 1000 mL
Diluent: Phosphate buffer solution pH 8.0 and methanol (60:40)
Phosphate buffer solution pH 7.0: Dissolve 35 g of dibasic potassium phosphate in 900 ml of water. Adjust to a pH of 7.0 with Diluted phosphoric acid and dilute with water to 1000 mL
Solution A: Phosphate buffer solution pH 7.0, water, and acetonitrile (5:60:35)
Solution B: Phosphate buffer solution pH 7.0, water, and acetonitrile (5:45:50)
Mobile phase: See Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| TRa | 100 | 0 |
| TR + 2 | 0 | 100 |
| TR + 15 | 0 | 100 |
TR = retention time of erythromycin B, determined by injecting 10 µL of Standard solution 2 and eluting with Solution A.
Standard solution 1: 4 mg/mL of USP Erythromycin BS in Diluent
Standard solution 2: 0.2 mg/mL of USP Erythromycin B. RS and USP Erythromycin C. RS in Diluent
Sample solution: 4 mg/mL of Erythromycin in Diluent
Chromatographic system
(See Chromatography (621) System Surtability)
Mode: LC
Detector: UV 210 nm
Column: 4.6-mm × 25-cm; 3.5-μm packing L1
Temperatures
Column: 65°, preheating the Mobile phase may be required, for instance by extending the inlet tubing in the oven to 30 cm
Sampler: 4°
Flow rate: 1.0 mL/min
Injection volume: 100 μL
System suitability
Sample: Standard solution 1
Suitability requirements
Tailing factor: NMT 2.0 for erythromycin A
Relative standard deviation: NMT 1.0% for erythromycin A, 6 replicate injections
Analysis
Samples: Standard solution 1, Standard solution 2, and Sample solution
Calculate the percentage of erythromycin A in the portion of Erythromycin taken:
Result = (rU/(rS) × (CS /CU) × P × 100
rU = peak response of erythromycin A from the Sample solution
rS = peak response of erythromycin A from Standard solution 1
CS = concentration of USP Erythromycin RS in Standard solution 1 (mg/mL)
CU = concentration of Erythromycin, calculated on the anhydrous basis, in the Sample solution (mg/mL)
P = percentage of erythromycin A in USP Erythromycin RS
Calculate the percentages of erythromycin B and erythromycin C in the portion of Erythromycin taken:
Result = (rU/(rS) × (CS /CU) × P × 100
rU = peak response of the relevant analyte from the Sample solution
rS = peak response of the relevant analyte from Standard solution 2
CS = concentration of the corresponding Reference Standard in Standard solution 2 (mg/mL)
CU = concentration of Erythromycin, calculated on the anhydrous basis, in the Sample solution (mg/mL)
P = potency of erythromycin B or erythromycin C in the corresponding Reference Standard (mg/mg)
Acceptance criteria
Sum of Erythromycin A, Erythromycin B, and Erythromycin C: 93.0%-102.0% on the anhydrous basis
Erythromycin B: NMT 5.0% on the anhydrous basis
Erythromycin C: NMT 5.0% on the anhydrous basis
3 IMPURITIES
RESIDUE ON IGNITION (283): NMT 0.2%
Change to read:
LIMIT OF THIOCYANATE
Use the Standard solutions, Sample solution, and Blank solution within 30 min.
Standard stock solutions 1 and 2: 0.2 mg/ml of potassium thiocyanate prepared in duplicate as follows. Transfer 100 mg of potassium thiocyanate, previously dried at 105" for 1 h and cooled, to a 50-ml, volumetric flask. Add about 20 ml of methanol to each flask, swirl to dissolve, and dilute with methanol to volume. Transfer 5.0 mL of this solution to a 50-mL volumetric flask and dilute with methanol to volume.
Standard solutions 1 and 2: 0.02 mg/mL, of potassium thiocyanate prepared in duplicate as follows. Transfer 5.0 mL of each of the Standard stock solutions to separate 50-ml low-actinic volumetric flasks, add 1.0 mL of ferric chloride TS, dilute with methanol to volume
Sample solution: 2 mg/mL of Erythromycin prepared as follows. Transfer 100 mg of Erythromycin to a 50-ml, low-actinic volumetric flask, add 20 ml. of methanol, and swirl to dissolve. Add 1.0 ml of ferric chloride TS and dilute with methanol to volume.
Blank solution: Add 1.0 ml. of ferric chloride TS to a 50-ml, low-actinic volumetric flask. Dilute with methanol to volume
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857))
Mode: UV-Vis
Analytical wavelength: 492 nm
System suitability
Samples: Standard solution 1, Standard solution 2, and Blank solution
Use the Blank solution to zero the instrument. Measure the absorbance of the two Standard solutions.
Suitability: 0.985-1.015
Calculate the suitability, S:
Result = (A1/W1) x (W2/A2)
A1 = absorbance of Standard solution 1
W1 = weight of the potassium thiocyanate taken to prepare Standard solution 1 (mg)
W2 = weight of the potassium thiocyanate taken to prepare Standard solution 2 (mg)
A2 = absorbance of Standard solution 2
Analysis
Samples: Standard solution 1, Standard solution 2, and Sample solution
Calculate the percentage of thiocyanate in the portion of Erythromycin taken:
Result = (Mr1/Mr2) x (Au/Wu)x 0.5 x [(W1/A1) + (W2/A2)]
Mr1 = molecular weight of thiocyanate, 58.08
Mr2= weight of potassium thiocyanate, 97.18
Au = absorbance of the Sample solution
Wu = weight of Erythromycin taken to prepare the Sample solution (mg)
W1 = weight of the potassium thiocyanate taken to prepare Standard stock solution 1 (mg)
A1 = absorbance of Standard solution 1
W2 = weight of the potassium thiocyanate taken to prepare Standard stock solution 2 (mg)
A2 = absorbance of Standard solution 2
Acceptance criteria: NMT 0.3%
Change to read:
ORGANIC IMPURITIES
Diluent, Solution A, Solution B, Mobile phase, Standard solution 1, Standard solution 2, Sample solution, and Chromatographic
system: Proceed as directed in the Assay. Prepare the erythromycin solutions immediately before use
Diluted standard solution: 0.04 mg/mL of USP Erythromycin RS prepared as follows. Dilute 1.0 ml of Standard solution 1 to a 100-ml volumetric flask and dilute with Diluent to volume
System suitability solution: Dissolve 4 mg of USP Erythromycin System Suitability Mixture RS in 1 ml of Diluent.
System suitability
Sample: System suitability solution
[NOTE-See Table 2 for the relative retention times. Use the reference chromatogram provided with USP Erythromycin System Suitability Mixture RS and the chromatogram obtained with System suitability solution to identify the specified impurity peaks. Use the chromatogram obtained with Standard solution 2 to identify erythromycin B and erythromycin C.]
Suitability requirements
Peak-to-valley ratio: NLT 1.5 for the ratio of the height of the pseudoerythromycin A enol ether peak to the height of the valley between the pseudoerythromycin A enol ether peak and the erythromycin B peak: NLT 2.0 for the ratio of the height of the erythromycin E peak to the height of the valley between erythromycin E peak and erythromycin A peak
Resolution: NLT 1.2 between 3"-N-demethylerythromycin A and erythromycin C
Analysis
Samples: Standard solution 2, Sample solution, and Diluted standard solution
Calculate the percentage of any individual impurity in the portion of Erythromycin taken:
Result = (rU/(rS) × (CS /CU) x P x (1/F) × 100
rU = peak response of any individual impurity (the peak other than erythromycin A, erythromycin B, and erythromycin C) from the Sample solution.
rS = peak response of erythromycin A from the Diluted standard solution
CS = concentration of USP. Erythromycin RS in the Diluted standard solution (mg/mL)
CU = concentration of erythromycin in the Sample solution (mg/mL)
P = percentage of erythromycin A in USP Erythromycin RS
F = relative responce factor (see Table 2)
Acceptance criteria: See Table 2. The reporting threshold is 0.2%.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Erythromycin A N-oxidea | 0.3 | 1 | 1.0 |
| Erythromycin Fb | 0.4 | 1 | 2.0 |
| 3”-N-Demethylerythromycin Ac | 0.5 | 1 | 2.0 |
| Erythromycin C | 0.55 | - | - |
| 3”-N-Demethyl-3”-N-formylerythromycin Ad | 0.63 | 9.1 | 0.4 |
| Erythromycin Ee | 0.9 | 1 | 3.0 |
| Erythromycin A | 1.0 | - | - |
| Anhydroerythromycin Af | 1.61 | 0.5 | 1.0 |
| Erythromycin B | 1.75 | - | - |
| Pseudoerythromycin A enol etherg | 1.81 | 12.5 | 1.0 |
| Erythromycin A enol etherh | 2.3 | 12.5 | 1.0 |
| Any other individual impurity | - | - | 0.4 |
| Total impurities | - | 7.0 |
a (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[(3,4,6-trideoxy-3-dimethylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione N-oxide.
b (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3-hydroxymethyl-5,7,9,11,13-pentamethyl-6-[(3,4,6-trideoxy-3-dimethylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione.
c (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[(3,4,6-trideoxy-3-methylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione.
d (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-Dideoxy-3-C-methyl-α-l-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[(3,4,6-trideoxy-3-formylmethylamino-β-d-xylo-hexopyranosyl)oxy]oxacyclotetradecane-2,10-dione.
e (2S,4aR,4’R,5’S,6’S,7R,8S,9R,10R,12R,14R,15R,16S,16aS)-7-Ethyl-5’,8,9,14-tetrahydroxy-4’-methoxy-4’,6’,8,10,12,14,16-heptamethyl-15-
{[3,4,6-trideoxy-3-(dimethylamino)-β-d-xylo-hexopyranosyl]oxy}hexadecahydrospiro[5H,11H-1,3-dioxino[5,4-c]oxacyclotetradecin-2,2’-pyrane]-5,11-dione.
f (1S,2R,3R,4S,5R,8R,9S,10S,11R,12R,14R)-9-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-5-ethyl-3-hydroxy-2,4,8,10,12,14-hexamethyl-11-{[3,4,6-trideoxy-3-(dimethylamino)-β-d-xylo-hexopyranosyl]oxy}-6,15,16-trioxatricyclo[10.2.1.11,4]hexadecan-7-one.
g (2R,3R,6R,7S,8S,9R,10R)-7-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-3-[(1R,2R)-1,2-dihydroxy-1-methylbutyl]-2,6,8,10,12-pentamethyl-9-[[3,4,6-trideoxy-3-(dimethylamino)-β-d-xylo-hexopyranosyl]oxy]-4,13-dioxabicyclo[8.2.1]tridec-1(12)-en-5-one.
h (2R,3R,4S,5R,8R,9S,10S,11R,12R)-9-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-l-ribo-hexopyranosyl)oxy]-5-ethyl-3,4-dihydroxy-2,4,8,10,12,14-hexamethyl-11-{[3,4,6-trideoxy-3-(dimethylamino)-β-d-xylo-hexopyranosyl]oxy}-6,15-dioxabicyclo[10.2.1]pentadec-1(14)-en-7-one.
4 SPECIFIC TESTS
Delete the following:
OPTICAL ROTATION (7815), Procedures, Specific Rotation, (USP 1-0-2024)
Change to read:
WATER DETERMINATION (921), Method
Sample solution: Use methanol containing 10% of imidazole in place of methanol in the titration vessel.
Acceptance criteria: NMT 6.5%
CHYSTALLINITY (695): Meets the requirements
5 ADDITIONAL REQUIREMENTS
Change to read:
PACKAGING AND STORAGE: Preserve in hermetic containers, and protect from light


