Ergotamine Tartrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
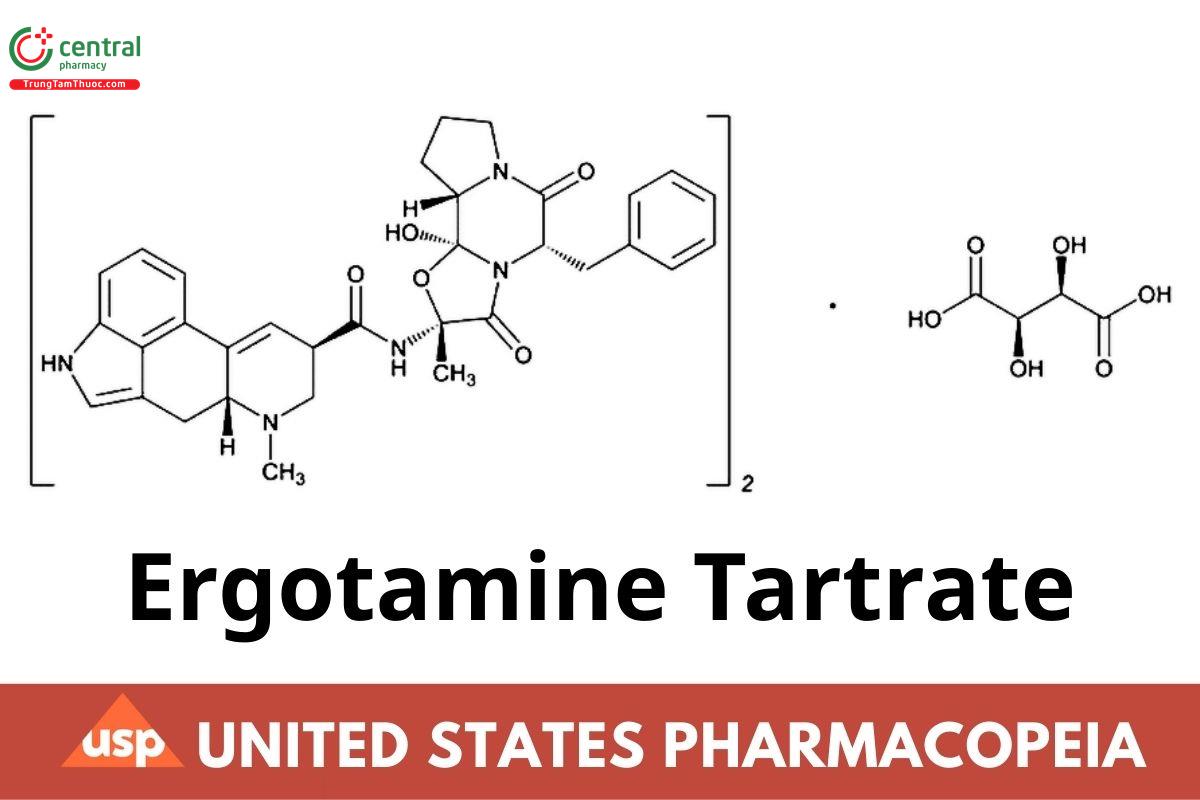
(C33H35N5O5)2 · C4H6O6 1313.41
Ergotaman-3′,6′,18-trione, 12′-hydroxy-2′-methyl-5′-(phenylmethyl)-, (5′α)-,[R-(R*,R*)]-2,3-dihydroxybutanedioate (2:1) (salt);
Ergotamine tartrate (2:1) (salt) CAS RN: 379-79-3; UNII: MRU5XH3B48
1 DEFINITION
Ergotamine Tartrate contains NLT 97.0% and NMT 100.5% of ergotamine tartrate [(C33H35N5O5)2 · C4H6O6], calculated on the dried basis.
2 IDENTIFICATION
A. The chromatogram of the Sample solution prepared as directed in the test for Related Alkaloids, exhibits its principal fluorescent spot and principal blue spot at the same R, values as the corresponding principle spots of the Standard solution.
3 ASSAY
PROCEDURE
Diluent: Acetic anhydride and glacial acetic acid (6:100)
Sample: 200 mg of Ergotamine Tartrate
Titrimetric system
Mode: Direct titration
Titrant: 0.05 N perchloric acid VS
Blank: 15 mL of Diluent
Endpoint detection: Visual
Analysis
Samples: Sample and Blank
Dissolve the Sample in 15 mL of Diluent. Add 1 drop of crystal violet TS, and titrate with Titrant from a 10-mL buret. Perform a blank determination, and make any necessary correction. Each mL of 0.05 N perchloric acid is equivalent to 32.84 mg of ergotamine tartrate (C33H35N5O5)2 · C4H6O6
Acceptance criteria: 97.0%~100.5% on the dried basis
4 IMPURITIES
RELATED ALKALOIDS
Conduct this test without exposure to daylight and with the minimum necessary exposure to artificial light.
Solution A: A mixture of 5.5 mL of hydrochloric acid and 4.5 mL of water
Diluent: Chloroform and methanol (90:10)
Standard solution: 10 mg/mL of USP Ergotamine Tartrate RS in Diluent
Diluted standard solutions: USP Ergotamine Tartrate RS from the Standard solution diluted with Diluent as listed in Table 1
Table 1
| Diluted standard solution | Concentration (mg/mL) | Percentage (%, for comparison with Sample) |
| A | 0.2 | 2.0 |
| B | 0.1 | 1.0 |
| C | 0.05 | 0.5 |
| D | 0.025 | 0.25 |
Sample solution: 10 mg/mL of Ergotamine Tartrate in Diluent
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel
Application volume: 5 µL
Developing solvent system: Ether, dimethylformamide, chloroform, and dehydrated alcohol (70:15:10:5)
Spray reagent: 200 mg of p-(dimethylamino)benzaldehyde in Solution A. Use a freshly prepared solution.
Analysis
Samples: Standard solution, Diluted standard solutions, and Sample solution
Line the chamber with filter paper, and allow it to equilibrate for 15 min. Place each spot over an opened bottle of ammonium hydroxide for 20 s, then allow the plate to dry in a current of cold air for 20 s. Develop the chromatogram until the solvent front has moved about 17 cm. Remove the plate from the developing chamber, allow the solvent to evaporate in a current of cold air for approximately 2 min, and spray with Spray reagent. Dry the plate at 60° for about 5 min, and compare the chromatograms.
Acceptance criteria: The RF value of the principal spot from the Sample solution corresponds to that from the Standard solution; the sum of the intensities of any secondary spots from the Sample solution is NMT the intensity of the principal spot from Diluted standard solution A; and the intensity of NMT one of the secondary spots is greater than that of the principal spot from Diluted standard solution B.
5 SPECIFIC TESTS
OPTICAL ROTATION, Specific Rotation(781S).
Solution A: 10 g/L of tartaric acid in water
Solution B: Use chloroform from which any alcohol present has been removed by prior washing with water
Sample solution A: Dissolve 350 mg of Ergotamine Tartrate in 25 mL of Solution A contained in a separator, then add 500 mg of sodium bicarbonate, and mix gently but thoroughly. Add 10 mL of Solution B, and shake vigorously. After the layers have separated, draw off the chloroform phase through a small filter, previously moistened with Solution B, into a 50-mL volumetric flask, Rapidly continue the extraction with three 10-mL portions of Solution B, passing the extracts through the same filter. Place the flask in a bath at 20° for 10 min. Dilute with
Solution B at 20° to 50.0 mL.
Sample solution B: Evaporate a 25.0-mL aliquot of Sample solution A on a rotary evaporator to dryness, maintaining the temperature of the bath below 45°. Dissolve the residue in 25 mL of glacial acetic acid.
Titrimetric system
Mode: Direct titration
Titrant: 0.05 N perchloric acid VS
Blank: 25 mL of glacial acetic acid
Endpoint detection: Visual
Analysis
Samples: Sample solution A, Sample solution B, and Blank
Add 1 drop of crystal violet TS to Sample solution B, and titrate with 0.05 N perchloric acid VS to an emerald-green endpoint. Perform a blank determination, make any necessary correction, and calculate the concentration of the ergotamine base. Each mL of 0.05 N perchloric acid is equivalent to 29.08 mg of ergotamine (C33H35N5O5)2
Determine the specific rotation of the ergotamine base from the angular rotation at 20° of Sample solution A and the concentration of the ergotamine base.
Acceptance criteria: -155° to-165°
LOSS ON DRYING (731).
Sample: 100 mg of Ergotamine Tartrate
Analysis: Dry the Sample under vacuum at 60° for 4 h.
Acceptance criteria: NMT 5.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers in a cold place.
USP Reference Standards 〈11〉
USP Ergotamine Tartrate R


