Ergocalciferol
If you find any inaccurate information, please let us know by providing your feedback here
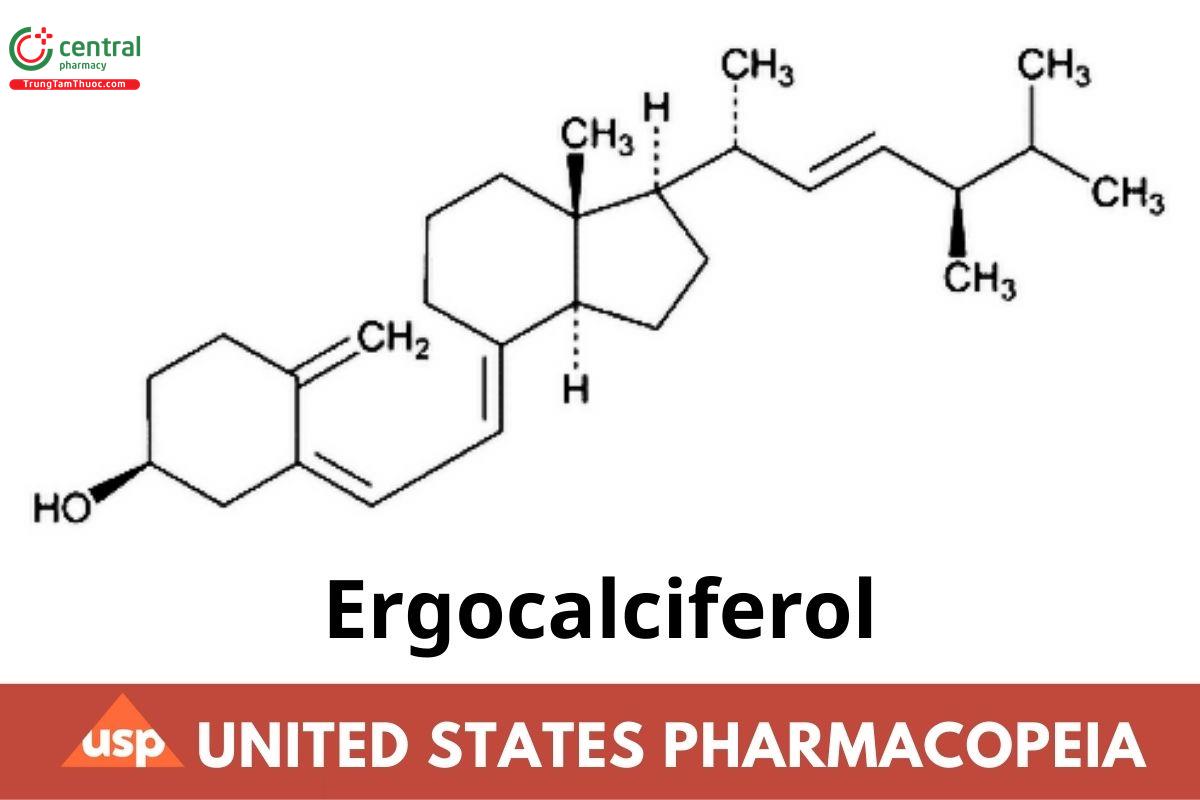
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C28H44O 396.65
9,10-Secoergosta-5,7,10 (19),22-tetraen-3-ol, (3β,5Z,7E,22E)-;
Ergocalciferol CAS RN: 50-14-6
1 DEFINITION
Ergocalciferol contains NLT 97.0% and NMT 103.0% of ergocalciferol (C28H44O).
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (USP 1-MAY-2020)
Wavelength range: 2-12 µm
Acceptance criteria: Meets the requirements in the chapter
Change to read:
B. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 1970 (USP 1-MAY-2020)
Analytical wavelength : 265 nm
Sample solution: 10 µg/mL in alcohol
Acceptance criteria: Meets the requirements in the chapter. Absorptivities do not differ by more than 3.0%.
Change to read:
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. (USP 1-May-2020)
Delete the following:
D. THIN-LAYER CHROMATOGRAPHY
[NOTE-For the Standard solutions and the Sample solution, follow these procedures: use low-actinic glassware, dissolve the samples without heating, and use the solutions immediately.]
Diluent: 10 mg/mL of squalane in chloroform
Standard solution A: 50 mg/mL of USP Ergocalciferol RS in Diluent
Standard solution B: 100 µg/mL of USP Ergosterol RS in Diluent
Sample solution: 50 mg/mL of Ergocalciferol in Diluent
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL.
Developing solvent system: Cyclohexane and ether (1:1)
Spray reagent: 20 mg/mL of acetyl chloride in antimony trichloride TS
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
[NOTE-Perform the development and subsequent operations in the dark.]
Place the plate in a chamber containing and equilibrated with Developing solvent system. Develop until the solvent front has moved about 15 cm above the line of application. Remove the plate, allow the solvent to evaporate, and spray with Spray reagent.
Acceptance criteria: The Sample solution shows a yellowish-orange area (ergocalciferol) having the same R, value as the area of Standard solution A and may show a violet area below the ergocalciferol area. The color of the violet area is not more intense than that of the violet area from Standard solution B (USP 1-May-2020)
3 ASSAY
Change to read:
PROCEDURE
(USP 1-May-2020)
Mobile phase: n-Amyl alcohol in hexane, solvent, chromatographic (USP 1-May-2020) (3 in 1000)
System suitability solution: 250 mg of USP Vitamin D Assay System Suitability RS in 10 mL of a mixture of toluene and Mobile phase (1:1).
Heat this solution, under reflux, at 90° for 45 min, and cool. [NOTE-This solution contains cholecalciferol, precholecalciferol, and trans-cholecalciferol.]
[NOTE-For the stock solutions, follow these procedures: use low-actinic glassware, dissolve the samples without heating, and prepare the solutions fresh daily.]
Standard stock solution: 0.6 mg/mL of USP Ergocalciferol RS in toluene
Standard solution: 120 µg/mL of USP Ergocalciferol RS in Mobile phase, prepared from Standard stock solution
Sample stock solution: 0.6 mg/mL of Ergocalciferol in toluene
Sample solution: 120 µg/mL of Ergocalciferol in Mobile phase, prepared from Sample stock solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm; packing 13
Flow rate: 1.5 mL/min (USP 1-May-2020)
Injection volume: 5-10 µL
System suitability
Sample: System suitability solution
[NOTE-The relative retention times for precholecalciferol, trans-cholecalciferol, and Cholecalciferol are 0.4, 0.5, and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.0 between trans-cholecalciferol and precholecalciferol
Relative standard deviation: NMT 2.0% for the peak response of cholecalciferol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ergocalciferol (C28H44O) in the portion of Ergocalciferol taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Ergocalciferol RS in the Standard solution (µg/mL)
Cu = concentration of Ergocalciferol in the Sample solution (µg/mL)
Acceptance criteria: 97.0%–103.0%
4 IMPURITIES
REDUCING SUBSTANCES
Standard solution: 0.2 µg/mL of Hydroquinone in dehydrated alcohol
Sample solution: 10 mg/mL of Ergocalciferol in dehydrated alcohol
Blank: Dehydrated alcohol
Analysis
Samples: Standard solution, Sample solution, and Blank
To 10 mL each of Standard solution, Sample solution, and Blank, add 0.5 mL of 5 mg/mL blue tetrazolium in methanol. Then add 0.5 mL of tetramethylammonium hydroxide TS in dehydrated alcohol (1 in 10). Allow the mixture to stand for 5 min, accurately timed, then add 1 mL of glacial acetic acid. Determine the absorbance of the solution at 525 nm, with a suitable spectrometer, against the Blank.
Acceptance criteria: The absorbance of the Sample solution is NMT that of the Standard solution.
5 SPECIFIC TESTS
MELTING RANGE OR TEMPERATURE (741), Procedures, Procedure for Class Ib. Apparatus / and Procedure for Class Ib. Apparatus II: 115-119o
OPTICAL ROTATION (781S), Procedures. Specific Rotation
Sample solution: 15 mg/mL in alcohol. (NOTE-Prepare and use the solution without delay. Use Ergocalciferol from a container opened not longer than 30 min.]
Acceptance criteria: +103° to r + 106 deg
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in hermetically sealed containers under nitrogen, and store in a cool place protected from light.
Change to read:
USP REFERENCE STANDARDS (11)
USP Ergocalciferol RS
(USP 1-May-2020)
USP Vitamin D Assay System Suitability RS


