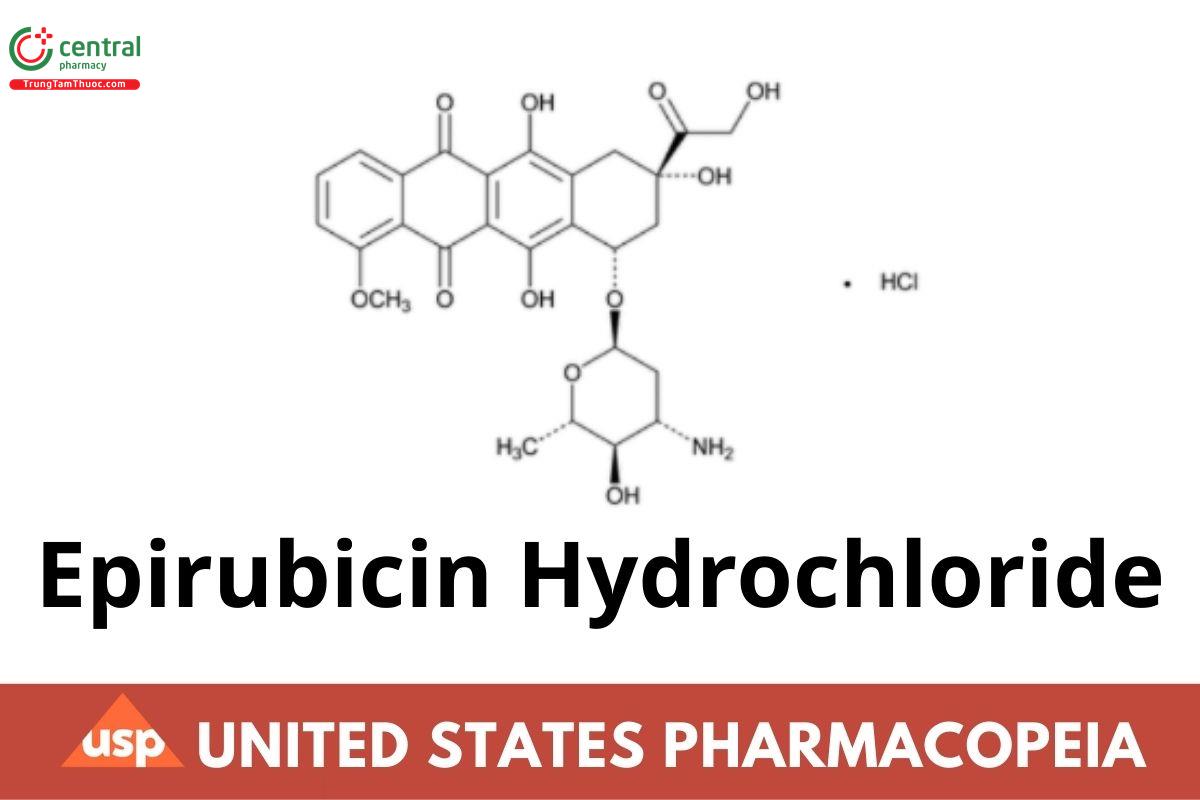
Epirubicin Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Epirubicin Hydrochloride contains NLT 97.0% and NMT 102.0% of epirubicin hydrochloride (C27H29NO11 · HCl), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. Identification Tests—General, Chloride〈191〉
Solution A: Nitric acid and water (1:1)
Sample solution: 10 mg/mL in Solution A
Acceptance criteria: Meets the requirements
3 ASSAY
Procedure
Allow the System suitability solution, Standard solution, and Sample solution to stand for 3 h before use.
Solution A: Dilute 10 mL of phosphoric acid with water to 100 mL.
Solution B:
Dissolve 3.7 g of sodium lauryl sulfate in 950 mL of water. To the resulting solution, add 28 mL of Solution A, and dilute with water to 1000mL.
Mobile phase: Acetonitrile, methanol, and Solution B (29:17:54)
System suitability solution: 0.1 mg/mL each of USP Epirubicin Hydrochloride RS and USP Doxorubicin Hydrochloride RS in Mobile phase
Standard solution: 1 mg/mL of USP Epirubicin Hydrochloride RS in Mobile phase
Sample solution: 1 mg/mL of Epirubicin Hydrochloride in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-μm packing L13
Column temperature: 35°
Flow rate: 2.5 mL/min
Injection volume: 10 μL
Run time: About 3.5 times the retention time of the epirubicin peak
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 2.0 between doxorubicin and epirubicin
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of epirubicin hydrochloride (C27H29NO11 · HCl) in the portion of Epirubicin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
P = potency of epirubicin hydrochloride in USP Epirubicin Hydrochloride RS (mg/mg)
Acceptance criteria: 97.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Organic Impurities
Allow the System suitability solution, Sample solution, and Standard solution to stand for 3 h before use.
Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Standard solution: 0.01 mg/mL of USP Epirubicin Hydrochloride RS in Mobile phase
Peak identification solution: Dissolve 10 mg of USP Doxorubicin Hydrochloride RS in 10 mL of a mixture of water and phosphoric acid (1:1).
Allow to stand for 30 min. Adjust with 2 N sodium hydroxide solution to a pH of 2.6. Add 15 mL of acetonitrile and 10 mL of methanol, and mix.
Analysis
Samples: Sample solution, Standard solution, and Peak identification solution
[Note—Use the Peak identification solution to identify the doxorubicinone peak.]
Calculate the percentage of each impurity in the portion of Epirubicin Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of epirubicin from the Standard solution
CS = concentration of USP Epirubicin Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Epirubicin Hydrochloride in the Sample solution (mg/mL)
P = potency of epirubicin hydrochloride in USP Epirubicin Hydrochloride RS (mg/mg)
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1. The reporting threshold is 0.05% of the area of the epirubicin peak in the Standard solution.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Doxorubicinonea | 0.3 | 1.4 | 1.0 |
| Daunorubicinoneb | 0.4 | 1.0 | 0.5 |
| Doxorubicin | 0.8 | 1.0 | 1.0 |
| Epirubicin | 1.0 | - | - |
Dihydro daunorubicinc | 1.1 | 1.0 | 0.5 |
| Daunorubicin | 1.5 | 1.0 | 0.5 |
| Epidaunorubicind | 1.7 | 1.0 | 1.0 |
| Epirubicin dimere | 2.1 | 1.0 | 1.0 |
Individual unspecified impurity | - | 1.0 | 0.5 |
| Total impurities | - | - | 3.0 |
a (8S,10S)-6,8,10,11-Tetrahydroxy-8-(hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
b (8S,10S)-8-Acetyl-6,8,10,11-tetrahydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
c Dihydrodaunorubicin; (8S,10S)-10-[(3-Amino-2,3,6-trideoxy-α-l-lyxo-hexopyranosyl)oxy]-6,8,11-trihydroxy-8-(1-hydroxyethyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione hydrochloride.
d (8S,10S)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-α-l-arabino-hexopyranosyl)oxy]-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione.
e 8,8′-[(2R,4R)-4-Hydroxy-2-(hydroxymethyl)-1,3-dioxolan-2,4-diyl]bis{(8S,10S)-10-[(3-amino-2,3,6-trideoxy-α-l-arabino-hexopyranosyl)oxy]-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione}.
Limit of Acetone
Analysis: See Residual Solvents 〈467〉.
Acceptance criteria: NMT 1.5%
5 SPECIFIC TESTS
Water Determination, Method Ic〈921〉: NMT 4.0%
pH 〈791〉
Sample solution: 5 mg/mL
Acceptance criteria: 4.0–5.5
Bacterial Endotoxins Test 〈85〉: NMT 1.1 USP Endotoxin Units/mg, where the label states that Epirubicin Hydrochloride is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Sterility Tests 〈71〉: Meets the requirements where the label states that Epirubicin Hydrochloride is sterile.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Store in airtight containers, protected from light. Store as per labeling instructions. Possible storage conditions could include the following: Store at a temperature between 2° and 8°. Store at room temperature. If the substance is sterile, store in a sterile, airtight, tamper-proof container.
USP Reference Standards 〈11〉
USP Doxorubicin Hydrochloride RS
USP Epirubicin Hydrochloride RS


