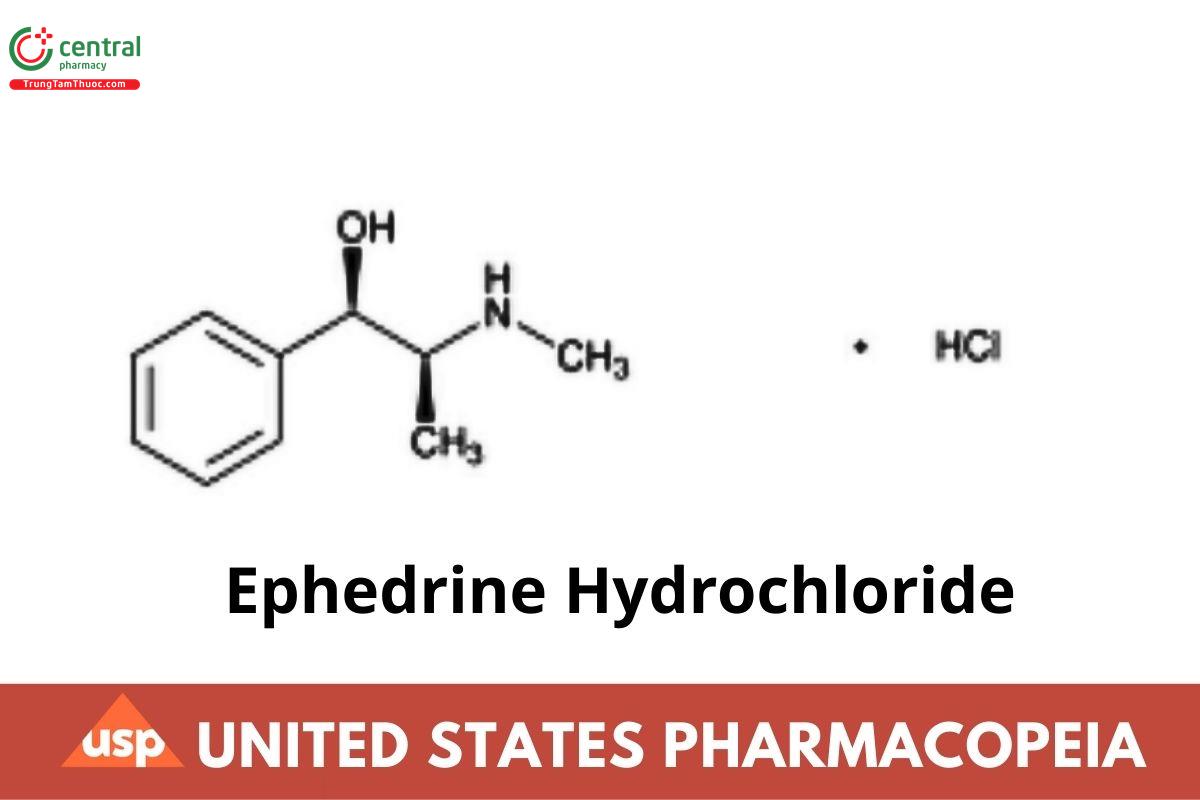
Ephedrine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Benzenemethanol, α-[1-(methylamino)ethyl]-, hydrochloride, [R-(R*,S*)]-;
(-)-Ephedrine hydrochloride;
(1R,2S)-2-(Methylamino)-1-phenylpropan-1-ol hydrochloride; CAS RN®: 50-98-6; UNII: NLJ6390P1Z.
1 DEFINITION
Ephedrine Hydrochloride contains NLT 98.0% and NMT 102.0% of ephedrine hydrochloride (C10H15NO· HCl),, calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A.SPECTROSCOEIC IDENTIFICATION TERTS (197), Infrared Spectroscopy: 197K (CN 1-May-2020)
B. IDENTIFICATION TESTS GENERAL (191), Chemical Identification Tests, Chloride: Meets the requirements
C. The retention time of the ephedrine peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Buffer: 11.6 g/L of ammonium acetate. Adjust with glacial acetic acid to a pH of 4.0.
Mobile phase: Methanol and Buffer (6:94)
Diluent: Methanol and water (6:94)
System suitability solution: 0.1 mg/ml each of LISP Ephedrine Hydrochloride RS and USP Pseudoephedrine Hydrochloride RS in Diluent
Standard solution: 0.2 mg/mL of USP Ephedrine Hydrochloride RS in Diluent
Sample solution: 0.2 mg/mL of Ephedrine Hydrochloride in Diluent
Chromatographic system
(See Chromatography (621), System Suitability)
Mode: LC
Detector: UV 257 nm
Column: 4.6-mm x 15-cm; 3-um packing L11
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 2 times the retention time of ephedrine
System suitability
Samples: System suitability solution and Standard solution
[Note-The relative retention times for ephedrine and pseudoephedrine are 1.0 and 1.1, respectively.]
Suitability requirements
Resolution: NLT 2.0 between ephedrine and pseudoephedrine, System suitability solution
Tailing factor: NMT 2.0 for ephedrine, Standard solution
Relative standard deviation: NMT 0.73% for ephedrine, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of ephedrine hydrochloride (C10H15NO· HCl) in the portion of Ephedrine Hydrochloride taken
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of ephedrine from the Sample solution
rS = peak response of ephedrine from the Standard solution
CS = concentration of USP Ephedrine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Ephedrine Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
RESIDUE ON JONITION (281): NMT 0.1%
CHLORIDE AND SULFATE (221), Sulfate
Sample solution: 1.25 mg/mL of Ephedrine Hydrochloride in water
Analysis: Add 1 ml. of 3 N hydrochloric acid and 1 ml. of barium chloride TS to 40 ml of the Sample solution.
Acceptance criteria: No turbidity develops within 10 min.
ORGANIC IMPURITIES
Buffer, Mobile phase, and Chromatographic system: Proceed as directed in the Assay, except for the Run time.
Run time: NLT 2.5 times the retention time of ephedrine
System suitability solution: 0.1 mg/mL each of USP Ephedrine Hydrochloride RS and USP Pseudoephedrine Hydrochloride RS in Mobile
phase
Sensitivity solution: 3.8 µg/mL of USP Ephedrine Hydrochloride RS in Mobile phase
Standard solution: 30 µg/mL of USP Ephedrine Hydrochloride RS in Mobile phase
Sample solution: 7.5 mg/ml of Ephedrine Hydrochloride in Mobile phase
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[NOTE-See Table 1 for the relative retention times.]
Suitability requirements
Resolution: NLT 2.0 between ephedrine and pseudoephedrine, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of e-acetylbenzyl alcohol or any unspecified impurity in the portion of Ephedrine Hydrochloride taken:
Result = (rU/(rS) × (CS /CU) × (1/F) ×100
rU = peak response of a acetylbenzyl alcohol or any unspecified impurity from the Sample solution
rS = peak response of ephedrine from the Standard solution
CS = concentration of USP Ephedrine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Ephedrine Hydrochloride in the Sample solution (mg/mL)
Frelative response factor (see Table 1)
Acceptance criteria: See Table 1. The reporting threshold is 0.05%
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Ephedrine | 1.0 | - | - |
| Pseudoephedrinea | 1.1 | - | - |
-Acetylbenzyl alcoholb | 1.4 | 2.5 | 0.2 |
| Any unspecified impurity | - | 1.0 | 0.1 |
| Total impurities | - | - | 0.5 |
a Included for identication only. It is not to be reported and not to be included in the total impurities.
b (-)-(1R)-1-Hydroxy-1-phenylpropan-2-one.
c Excludes a-acetylbenzyl alcohol.
5 SPECIFIC TESTS
OPTICAL ROTATION (7815), Procedures. Specific Rotation
Sample solution: 50 mg/mL of Ephedrine Hydrochloride in water
Acceptance criteria: −33.0° to −35.5°
LOSS ON DEYING (731)
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 0.5%
ACIDITY OR ALKALINITY
Sample solution: 50 mg/ml. of Ephedrine Hydrochloride in water
Analysis: To 20 mL of Sample solution add 1 drop of methyl red TS.
Acceptance criteria: if the solution is yellow, it is changed to red by NMT 0.10 mL of 0.020 N sulfuric acid. If the solution is pink, it is changed to yellow by NMT 0.20 mL of 0.020 N sodium hydroxide.
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed, light-resistant containers.


