Enzacamene
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
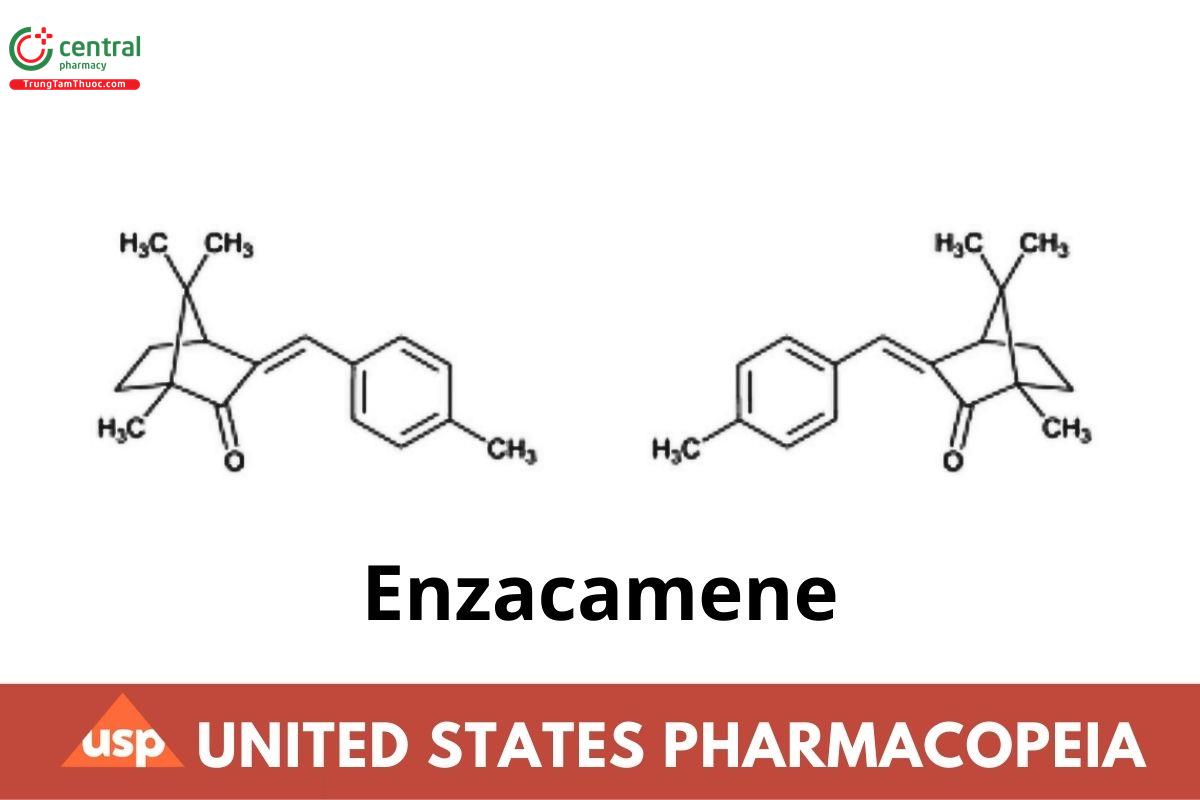
Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-3-[(4-methylphenyl)methylene]-;
(±)-3-(p-Methylbenzylidene)camphor;
4-Methylbenzylidene camphor;
1,7,7-trimethyl-3-(4-methylbenzylidene)bicyclo[2.2.1]heptan-2-one CAS RN®: 36861-47-9; UNII: 8I3XWY40L9.
1 DEFINITION
Enzacamene contains NLT 98.0% and NMT 102.0% of enzacamene (C18H22O), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscop) 197A or (USP 1-Dec-2022) 197K
B. SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 197U
Sample solution: 10 µg/mL in methanol
Acceptance criteria: Meets the requirements
3 ASSAY
Change to read:
PROCEDURE
Standard solution: 250 µg/ml. each of Camphor and USP Enzacamene Enzacamene RS (USP 1-Dec-2022) in methylene chloride (USP 1-Dec-2022)
Sample solution: 250 µg/ml, of Enzacamene in methylene chloride (USP 1-Dec-2022)
Chromatographic system
(See Chromatography (621), System Suitability)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m fused-silica capillary; coated with a 0.25-μm layer of phase G1
Temperatures
Injection port: 295°
Detector: 305°
Column: See Table 1. The column temperature is maintained at 300" until the end of the procedure.
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 100 | - | 100 | 5 |
| 100 | 10 | 230 | 10 |
| 230 | 10 | 300 | - |
Carrier gas: Helium
Flow rate: 1.2 ml/min
Injection volume: 1μL
System suitability
Bample: Standard solution
Suitability requirements
Tailing factor: NMT 10 for encacamene
Relative standard deviation: NMT 2.0% for tamete
Analysis
Samples: Standee solution and Sample solution
Calculate the percentage of eczacamene (C18H22O) in the portion of Exzacamere taken
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of enzacamene from the sample solution
rS = peak response of enzacamene from the Standard solution
CS = concentration of USP Escocamins RS the Standardson solution (mg/ml)
CU = concentration of ncacamene in as the 2022) the Sample solution (mg/m)
Acceptance criteria: 98.0%-102.01% on the dined basis
4 IMPURITIES
Change to read:
Standard solution, Sample solution, Chromatographic system, and lystem suitability: Proceed as directed in the Assay
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Enzacamene taken
Result = (rU/(rS) x 100
rU = peak response of each impurity from the sample solution
rS = peak response of enzacamene from the Standard solution
Acceptance criteria: See Table 2
| Name | Relative Retention Time | Acceptance Criberla NMT (%) |
| Camphora | 0.2 | 0.02 |
| Enzacamene | 1.0 | - |
| Any | - | 0.1 |
| - | 0.5 |
a (1R,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one.
5 SPECIFIC TESTS
Loss on Drying (731)
Analysis: Dry under vacuum at 50° to constant weight.
Acceptance criteria: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
PARAD Sto: Preserve in tight containers.


