Entecavir
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
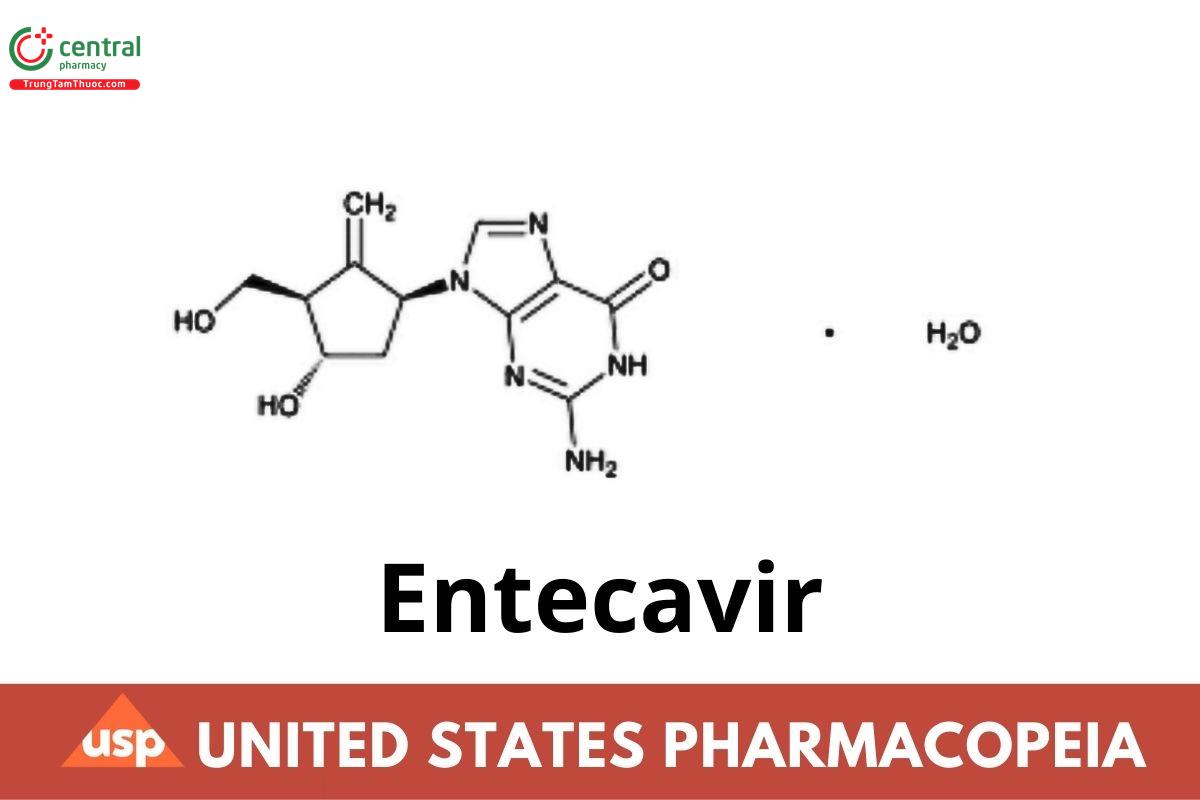
6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-, monohydrate;
9-[(1S,3R,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine monohydrate CAS RN®: 209216-23-9; UNII: 5968Y6H45M.
Anhydrous 277.28
1 DEFINITION
Entecavir is a monohydrate and contains NLT 98% and NMT 102% of entecavir (C12H15N5O3), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A.SPECTROSCOPIC IDENTIFICATION TESTA (197), Infrared Spectroscopy: 197A or 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Solution A: Acetonitrile and water (3:97)
Solution B: Acetonitrile
Mobile phase: See Table 1. (Note-The gradient elution times are established on an HPLC system with a dwell volume of approximately 1.0 mL]
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 8 | 100 | 0 |
| 50 | 77 | 23 |
| 75 | 17 | 83 |
| 90 | 100 | 0 |
| 100 | 100 | 0 |
System suitability stock solution: 1.0 mg/ml, of USP Entecavir System Suitability Mixture RS in methanol
System suitability solution: 0.2 mg/mL of USP Entecavir System Suitability Mixture RS in Solution A from System suitability stock solution
Standard stock solution: 1.0 mg/mL of USP Entecavir Monohydrate RS in methanol. Sonicate as needed.
Standard solution: 0.2 mg/mL of USP Entecavir Monohydrate RS in Solution A from the Standard stock solution
Sample stock solution: 1.0 mg/mL of Entecavir in methanol. Sonicate as needed.
Sample solution: 0.2 mg/ml. of Entecavir in Solution A from Sample stock solution
Chromatographic system
(See Chromatography (621) System Suitability)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm, 5-um packing L1
Flow rate: 1 mL/min
Injection volume: 10 μL
System suitability
Samples: System suitability solution and Standard solution
[Note-See Table 2 for the relative retention times of the components in the System suitability solution.]
Suitability requirements
Resolution: NLT 3.5 between entecavir 1-epimer and entecavir, NLT 2.0 between entecavir and 8-hydroxy entecavir, System suitability solution
Tailing factor: 0.8-1.5 for entecavir, System suitability solution
Relative standard deviation: NMT 1.59%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of entecavir (C12H15N5O3) in the portion of Entecavir taken:
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of entecavir from the Sample solution
rS = peak response of entecavir from the Standard solution
CS = concentration of USP Entecavir Monohydrate RS in the Standard solution (mg/ml)
CU = concentration of Entecavir in the Sample solution (mg/mL)
Acceptance criteria: 98%-102% on the anhydrous basis
4 IMPURITIES
ORGANIC INPURITIES
Solution A, Solution B, Mobile phase, System suitability stock solution, System suitability solution, Sample stock solution, Sample
solution, and Chromatographic system: Proceed as directed in the Assay.
Standard stock solution: Use the Standard solution from the Assay.
Standard solution: 0.2 µg/ml. of USP Entecavir Monohydrate RS in Solution A from the Standard stock solution.
System suitability
Samples: System suitability solution and Standard solution
[Note-See Table 2 for relative retention times of the components in the System suitability solution]
Suitability requirements
Resolution: NLT 3.5 between entecavir 1-epimer and erntecavir, NLT 2.0 between entecavir and 8-hydroxy entecavir, System suitability solution
Tailing factor: 0.8-1.5 for entecavir, System suitability solution
Relative standard deviation: NMT 10.0%, Standard solution
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of each impurity in the portion of Entecavir taken:
Result = (rU/(rS) × (CS /CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of entecavir from the Standard solution
CS = concentration of USP Entecavir Monohydrate RS in the Standard solution (mg/mL)
CU = concentration of Entecavir in the Sample solution (mg/mL)
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2. Disregard any peak less than 0.05%
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Furoentecavira | 0.73 | 1.0 | 0.1 |
| Entecavir 1-epimerkb | 0.93 | 1.0 | 0.1 |
| Entecavir 3-epimerc | 0.96 | 1.0 | 0.1 |
| Entecavir | 1.0 | - | - |
| 8-Hydroxy entecavird | 1.03 | 0.67 | 0.1 |
| Entecavir 4-epimere | 1.08 | 1.0 | 0.1 |
| 8- Methoxy entecavirf | 1.27 | 0.67 | 0.1 |
| 4-Dimethylsilyl entecavirg | 1.84 | 1.0 | 0.1 |
| Entecavir related compound A | 3.41 | - | -h |
| Any unspecified impurity | - | 1.0 | 0.1 |
| Total impurities | - | - | 0.3 |
a 9-[(3aS,4S,6S,6aR)-3a,6-Dihydroxyhexahydro-1H-cyclopenta[c]furan-4-yl]guanine.
b 9-[(1R,3R,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
c 9-[(1S,3S,4S)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
d 8-Hydroxy-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
e 9-[(1S,3R,4R)-4-Hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
f 8-Methoxy-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
g 9-[(1S,3R,4S)-4-Hydroxydimethylsilyl-3-(hydroxymethyl)-2-methylenecyclopentyl]guanine.
h For information only; quantitated in the test for Limit of Entecavir Related Compound A.
i Includes the sum of all the impurities found in the tests for Limit of Entecavir Related Compound A and Organic Impurities.
LIMIT OF ENTECAVIR RELATED COMPOUND A
Solution A: 0.1% (v/v) trifluoroacetic acid in water
Solution B: 0.1% (v/v) trifluoroacetic acid in acetonitrile
Mobile phase: See Table 3. Nor-The gradient elution times are established on an HPLC system with a dwell volume of approximately 1.0 mL]
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 65 | 35 |
| 8 | 53 | 47 |
| 8.1 | 65 | 35 |
| 11 | 65 | 35 |
Standard solution: 2 μg/mL of USP Entecavir Related Compound A RS in methanol
Sample solution: 1.0 mg/mL of Entecavir in methanol. Sonicate as needed.
Chromatographic system
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 5-cm; 5-μm packing L1
Temperatures
Autosampler: 4°
Column: 30°
Flow rate: 2 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: 0.8–1.5
Relative standard deviation: NMT 3.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of entecavir related compound A in the portion of Entecavir taken:
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of entecavir related compound A from the Sample solution
rS = peak response of entecavir related compound A from the Standard solution
CS = concentration of USP Entecavir Related Compound A.RS in the Standard solution (mg/mL)
CU = concentration of Entecavir in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.1%
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method I. Method Ic: 5.5%-7.0%
OPTICAL ROTATION (7815), Procedures. Specific Rotation
Sample solution: 10 mg/mL of Entecavir in a mixture of dimethylformamide and methanol (50:50)
Acceptance criteria: +24° to +30°
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers, protected from light. Store at room temperature.


