Enrofloxacin
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
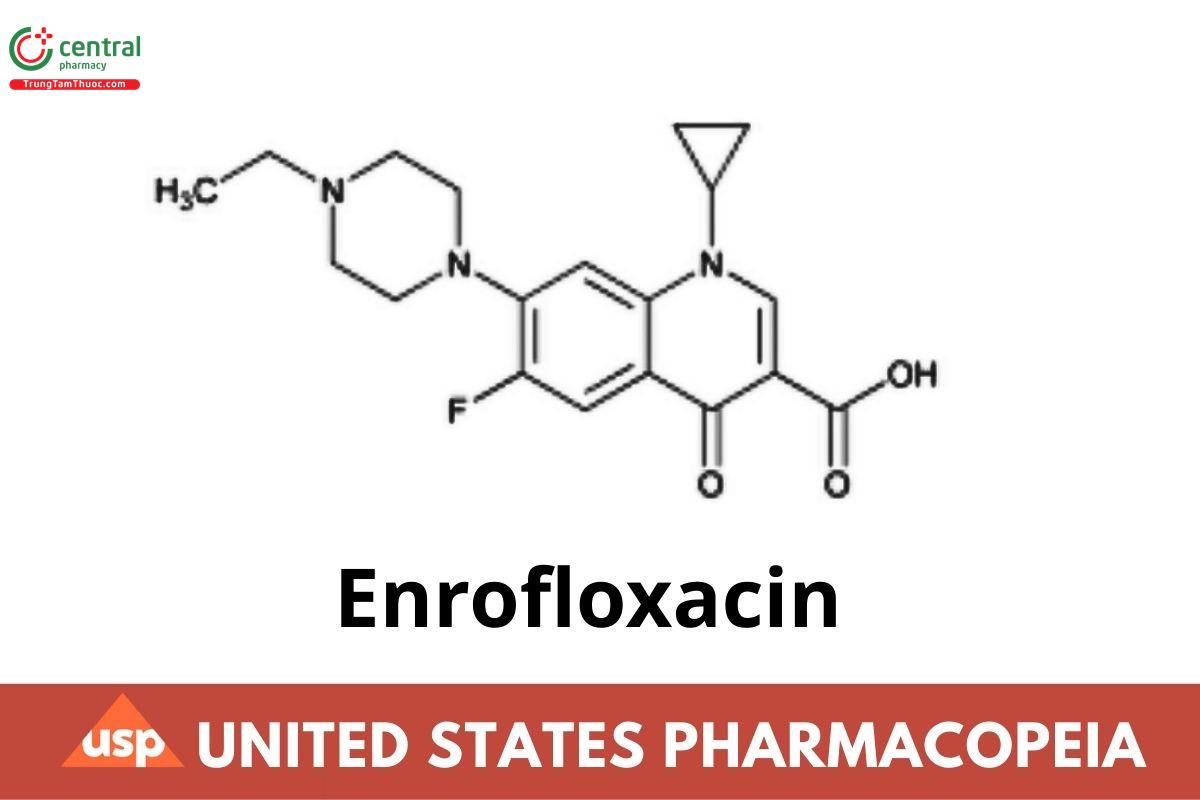
3-Quinolinecarboxylic acid, 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-uoro-1,4-dihydro-4-oxo-;
1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-uoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid CAS RN®: 93106-60-6; UNII: 3DX3XEK1BN.
Enrofloxacin contains not less than 98.5 percent and not more than 101.5 percent of C19H22FN3O3, calculated on the dried basis.
Packaging and storage—Preserve in well-closed, tight, light-resistant containers.
Labeling—Label it to indicate that it is for veterinary use only.
Contains a mixture of USP Enrofloxacin RS, desfluoro-enrofloxacin, and USP Ciprofloxacin RS.
Desfluoro-enrofloxacin 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-1,4-dihydro-4-oxo-3-quinoline-carboxylic acid.
Change to read:
CLARITY OF SOLUTION—
Hydrazine sulfate solution-Transfer 1.0 g of hydrazine sulfate to a 100-ml, volumetric flask, dissolve in and dilute with water to volume, and mix. Allow to stand for 4 to 6 hours.
Hexamethylenetetramine solution-Transfer 2.5 g of hexamethylenetetramine to a 100-mL, volumetric flask, add 25.0 mL of water, insert a glass stopper, and mix to dissolve.
Primary opalescent suspension—[Note—This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well-mixed before use.) Transfer 25.0 mL of the Hydrazine sulfate solution to the Hexamethylenetetramine solution in the 100-ml glass-stoppered flask. Mix, and allow to stand for 24 hours.
Opalescence standard Nor-This suspension should not be used beyond 24 hours after preparation.]
Transfer 15.0 ml of the Primary opalescent suspension to a 1000-mi volumetric flask, dilute with water to volume, and mix.
Reference suspension-Transfer 10.0 mL of the Opalescence standard to a 100-ml volumetric flask, dilute with water to volume, and mix, Test solution-To 1.0 g of Enrofloxacin add about 0.25 g of potassium hydroxide and 7 ml, of water. Sonicate to dissolve, and dilute with water to 10.0 ml..
Procedure—Transfer a sufficient portion of the Test solution, the Reference suspension, and water to separate test tubes of colorless transparent, neutral glass with a flat base and an internal diameter of 15 to 25 mm to obtain a depth of 40 mm. Compare the Test solution, the Reference suspension, and water in diffused daylight 5 minutes after preparation of the Reference suspension, viewing vertically against a black background (see Visual Comparison (630)). (Nore-The diffusion of light must be such that the Reference suspension can be readily distinguished from water.] The Test solution shows the same clarity as that of water or its opalescence is not more pronounced than that of the Reference suspension.
Change to read:
COLOR OF SOLUTION—
Standard stock solution-Combine 9.6 ml. of ferric chloride CS, 0.2 ml of cobaltous chloride CS, and 0.2 ml. of cupric sulfate CS, and mix Standard solution-[Note-Prepare the Standard solution immediately before use.] Transfer 5.0 mL of the Standard stock solution to a 100-ml
volumetric flask, and dilute with dilute hydrochloric acid (10 g per 1000 mL).
Test solution-To 1.0 g of Enrofloxacin add about 0.25 g of potassium hydroxide and 7 mL of water. Sonicate to dissolve, and dilute with water to 10.0 ml.
Procedure-Transfer a sufficient portion of the Test solution, the Standard solution, and water to separate test tubes of colorless, transparent, neutral glass with a flat base and an internal diameter of 15 mm to 25 mm to obtain a depth of 40 mm. Compare the Test solution, the Standard solution and water in diffused daylight, viewing vertically against a white background (see (3) ON 1 May 2019) Nur-The diffusion of light must be such that the Reference suspension can be readily distinguished from water. The Test solution has The appearance of water or is not more intensely colored than the Standard solution
Identification—
Change to read
A: Spectroscopic Identification Tests (197), Infrared Spectroscopy: 197K.
B: Thin-Layer Chromatographic Identification Test (201)—
Teat solution: Prepare a solution containing 10 mg of Enrofloxacin per ml of 0.05 Mt hydrochloric acid
Application volume: 5 μL.
Developing solvent solution methylene chloride, methanol, 25% ammonia solution, and acetonitrile (2:2:1:05)
Procedure— Proceed as directed in the chapter except apply 5-ul portions of the Test solution and the Standard solution to the starting line of the chromatographic plate. Place the plate in an atmosphere of ammonia far approximately 15 minutes. Develop the chromatogram in an unsaturated.chamber with the Developing solvent solution
Loss on drying (713)—Dry a 2-g sample, accurately weighed 5 to 7 hours under vacuum at 120 to constant weight it loses not more than 1.0% of its weight.
Residue on ignition (281): not more than 0.1%, based on a text specimen of about 2 g
Change to read:
Chloride 211—Add 50 ml of water to 0.5 g of Enrofloxacin, shake for 5 minutes, and pass through a chloride-hee filter paper, add 2 ml of 2 M acetic acid, and mix. Transfer 15.0 ml of the fiftrate to a 30-ml color-comparison bibe (Test solution. To a second metched 50-ml color comparison tube transfer 10.10 ml of a Standard solution of sodium chloride having a concentration of 8.2 µg per 1912, corresponding to 5 µg of chloride per ml, add 5.0 ml of water, and mic. To each tube add 1 ml of 2 N nitric acid, mix, add 1 mi, of silver nitrate TS, and mix. Allow the solutions to stand for 5 minutes, protected from light. Examine the tubes vertinally agarust a black background (see Visual Comparison 5
Any opalescence in the Test solution is not more intense than that in the Standard solution (0.04%
Change to read:
Dissolve 0.5 g of Enrofloxacin in 5.0 ml. of 2 N acetic acid and 15.0 ml of water (Test solution). To each of two 50 ml matched color-comparison tubes transter 1.50ml, of a Standard solution of potassium sulfate in 30% alcohol having a concentration of 18.1 ug per mi equivalent to 10 ug of sulfate per mi. To each tube add, successively and with continuous shaking, 1.0 ms of barium chloride solution (1 in) and allow to stand for 1 minute. To one of the tubes transfer 15.0 mL of the Standard solution and 0.5 ml of 30% acetic acid and max. To the second tube add 150 ml of the Test solution and 0.5 ml. of 30% aceti anit, and mix. Allow solutions to stand for 5 minutes Examine the tubes vertically against a black background bee Visual Common 1630 intense than that in the Standardl soon (0.04%) -2019). Any opalescence in the Test anlation is not more
Limit of N-ethylpiperazine—
Intermal standard solution-Dissolve an accurately weighed quantity of in decane in chloroform, and dilute quantitatively, and stepwise f necessary, with chloroform to obtain a solution having a known concentration of about 0.1 mg per ml
Stimand stock solution-Dissolve an accurately weighed quantity of USP N-Ethylpiperazine RS in chloroform, and dilute quantitatively, and stepwise if necessary, with chloroform to obtain a solution having a known concentration of about 9.0 mg per m
Stendard solution To 2.0 mL of the Intemal standard solution adid 20 µL of the Standard stock solution, and mox.
Tiestoon-To 200 mg of Errafloxacin, accurately weighed add 2.0 ml of the internal standard solution, and mix.
Chromatographic system (see Game (6211)-The gas chromatograph is equipped with a flame-ionization detector and a split injector system and contains a 0.32mm x 50m column with 100% liquid phase G1 with a film thickness of about 5.0 um. The carrier gas is hydrogen
(helium may be used), flowing at a rate of about 2.9 ml, per minute. The suxiliary gas is strogen flowing at a rate of about 30 ml. per minute The chromatograph is programmed as follows. Initially the temperature of the column is equilibrated at 80", then the temperature is increased at a tate of 10 per menute to 240", and maintained at 240 for 15 minutes. The split injector (25.1 split natio) temperature is maintained at 200", and the detector is maintained at 250. Chromatograph the Standard solution, and record the peak responses as directed for Procedure the relative ninention times are about 0.90 for Nethylpiperazine and 1.0 for decane
Procedure-Inject a volume (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatogram, and measure the responses for the major pasko. Calculate the percentage of the impurity in the portion of Enrofloxacin taken by the formula
100(Cs/Cu)(Ru/Rs)
in which is the concentration of the impunity, in mg per mil in the Standard solution: C, is the concentration, in mg per mil, of Enrofloxacin in the Test solutior is the peak response ratio of the impurity peak to the internal standard peak obtained from the Test solution, and R is the pesk response ratio of the impurity peak to the internal standard peak obtained from the Standard solution. Not more than 0.1% of the impurity is found
Related compounds—
TEST 1 FOR FLUEROQUINOLONIC ACID)—
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture.
Test solution—Prepare as directed for the Test solution in Identification test B.
Diluent—Transfer 0.1 ml, of 6 M ammonium to a 100-ml volumetric flask, mix, and dilute with water to volume.
Standard stock solution—Dissolve and mix an accurately weighed quantity of USP Fluoroquinolonic Acid RS with Diluent to prepare a solution containing about 0.10 mg per ml, and mix.
Standard solution 1 (0.1%)-Transfer 1.0 mL of the Standard stock solution to a 10-ml volumetric flask, dilute with water to volume, and mix.
Standard solution 2 (0.2%)-Transfer 2.0 mL of the Standard stock solution to a 10-ml volumetric flask, dilute with water to volume, and mix.
Application volume: 5 µL.
Developing solvent system—[Note-Carefully follow the mixing order stated below. Shake butyl acetate, n-butanol, water, and glacial acetic acid (50-9:15:25), and allow to settle. Use the upper layer as the mobile phase and discard the lower layer
Procedure—Proceed as directed for Thin-Layer Chromatography under Chromatography (621), Apply separately the Test solution, Standard solution 1, and Standard solution 2 to the thin-layer plate, and chromatograph using the Developing solvent system. Dry the developed chromatogram in the air under a fume hood for 30 to 60 minutes, then view under short-wavelength UV light. Determine the quantity of fluoroquinolonic acid by comparing the size and intensity of the spots from the Test solution to the Standard solutions. The intensity of any spot from the Test solution at about the same retardation factor, R,, as that of the Standard solutions is not greater than the intensity of the
Standard solution 2 (0.2%) spot.
TEST 2 (FOR CIPROFLOXACIN, DES-FLUORO COMPOUND AND OTHER UNSPECIFIED IMPURITIES)—
Phosphoric acid buffer—Prepare 25 mM phosphoric acid, and adjust with triethylamine to a pH of 3.0.
Mobile phase—Prepare a solution containing Phosphoric acid buffer and acetonitrile (87:13).
Control solution—Dissolve about 5 mg of USP Enrofloxacin Related Compound Mixture RS, accurately weighed, in Mobile phase in a 5-ml volumetric flask, dilute with Mobile phase to volume, and mix.
Test solution 1—-Dissolve about 50 mg of Enrofloxacin, accurately weighed, in Mobile phase in a 50-ml volumetric flask, dilute with Mobile phase to volume, and mix.
Test solution 2—Transfer 1.0 ml. of Test solution 1 into a 50-ml, volumetric flask, dilute with Mobile phase to volume, and mix. Transfer 1.0 ml of this solution into a 10-ml volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system (see CHROMATOGRAPHY (621))- The liquid chromatograph is equipped with a 278-nm detector and a 4.6-mm x 25-cm stainless steel column that contains 5-um packing L1. The column temperature is maintained at 40", and the flow rate is about 1.5 ml per minute. Chromatograph the Control solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.58 for the des-fluoro compound, 0.74 for ciprofloxacin, and 1,0 for enrofloxacin. The resolution, R, between the des fluoro compound and ciprofloxacin is not less than 1.5.
Procedure-Inject a volume (about 25 µL) of Test solution 1, Test solution 2, and the Control solution into the chromatograph, record the chromatogram, and measure the peak responses. Identify the ciprofloxacin and the des-fluoro compound peaks in Test solution 2 by comparing their retention times with those from the Control solution. Calculate the percentage of each related compound in the portion of Enrofloxacin taken by the formula:
100C(ri/rs)
in which C is the concentration of Enrofloxacin in Test solution 2 as a percentage of Test solution 1 (0.2%); ri is the individual peak response of each related compound obtained from Test solution 1; and rs is the individual peak area of enrofloxacin obtained from Test solution 2: not more than 0.1% of des-fluoro compound, not more than 0.3% of ciprofloxacin, not more than 0.1% of any unspecified impurity, and not more than 0.5% of total impurities are found.
Assay—Transfer about 250 mg of Enrofloxacin, accurately weighed, to a 125-mL flask, dissolve in 100 ml. of anhydrous acetic acid, and titrate with 0.1 M perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 M perchloric acid is equivalent to 35.94 mg of C19H22FN3O3.


