Enalapril Maleate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
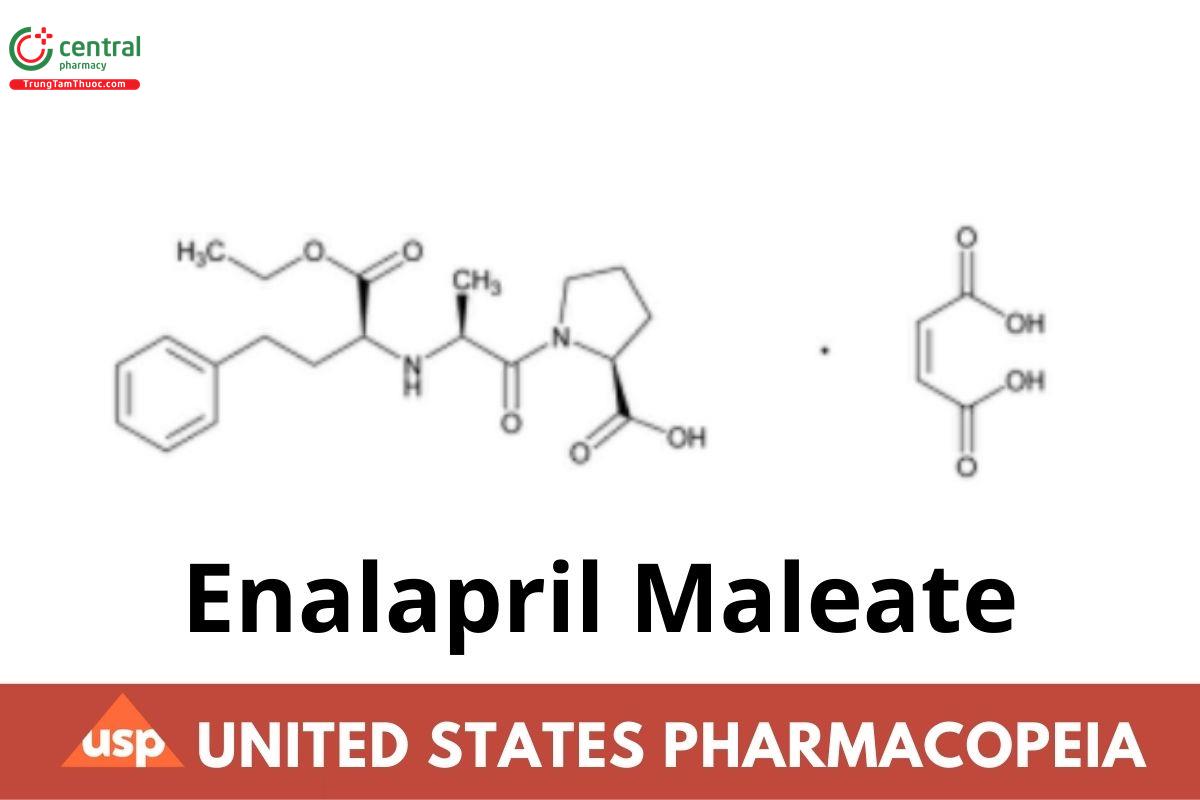
l-Proline, 1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-l-alanyl]-, (S)-, (Z)-2-butenedioate (1:1);
1-[N-[(S)-1-Carboxy-3-phenylpropyl]-l-alanyl]-l-proline, 1′-ethyl ester, maleate (1:1) CAS RN®: 76095-16-4; UNII: 9O25354EPJ.
1 DEFINITION
Enalapril Maleate contains NLT 98.0% and NMT 102.0% of enalapril maleate (C20H28N2O5 · C4H4O4) calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A .SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: (197M) or (197A)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay
3 ASSAY
Change to read:
PROCEDURE
Buffer 1: 2.8 g of sodium phosphate, monobasic in 900 ml. of water in a 1000-ml volumetric flask. Adjust with a 9 M sodium hydroxide A
Buffer 2: 2.8 g of sodium phosphate, monobasic in 900 ml of water in a 1000-mL volumetric flask. Adjust with phosphoric acid to a pH of (USP 1-Dec-2019) 2.5, and dilute with water to volume.
Solution A: Acetonitrile and Buffer 7 (5:95)
Solution B: Acetonitrile and Buffer 1 (66:34)
Mobile phase: See Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 95 | 5 |
| 20 | 40 | 60 |
| 25 | 40 | 60 |
| 26 | 95 | 5 |
| 30 | 95 | 5 |
Diluent: Acetonitrile and Buffer 2 (5.95)
Standard solution: 0.3 mg/ml of USP Enalapril Maleate RS in Diluent
Sample solution: 0.3 mg/ml. of Enalapril Maleate in Diluent
Chromatographic system (See Chromatography (621) System Suitability)
Mode: LC
Detector: sqrt(215nm)
Columns: 4.1mm x 15cm packing L
Column temperature: 70°
Flow rate: 1.5 l/min
Injection volume: 50
System suitability
Sample: Standard solution 1 De-201
Suitability requirements
*Talling factor: NMT 2.0
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standardson and Sample solution
Calculate the percentage of enalapril maleate (C20H28N2O5 · C4H4O4) in the portion of Enalapril Maleste taken
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of enalapril from the Sample solution
rS = peak response of enalapril from the Standard solution
CS = concentration of USP Enalapril Maleate RS in the Standard solution (mg/ml)
CU = concentration of Enalapril Maleate in the Sample solution (mg/ml)
Acceptance criteria: 98.0%-102.01% on the dead basis
4 IMPURITIES
Residue on Ignition (281): NMT 0.2%
Change to read
Buffer: 0.14 g/l, of notassum choentate mensbasis in wates Adjust with abouabionic acid to a pH of 2.0
Solution A Acetustzile and Buffer (1090)
Solution B: Acetonitrile and Buffer (80:20)
Mobile phase: See Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 5 | 100 | 0 |
| 21 | 80 | 20 |
| 30 | 15 | 85 |
| 40 | 100 | 0 |
| 50 | 100 | 0 |
Diluent Acctuntile and Buffer (20:80)
System suitability solution: 1 mg/m of USP Enalapril Maleate RS and 3 ugimi of USP Moexpril Related Compound F-RG in Diluent, Sonicate to dissolve as needed.
Standard solution: 0.01 mg/mL of USP Enalapril Maleate RS in Diluent
Sensitivity solution: 0.5 µg/m of USP Enalapril Maleate RS in Ditwns from the Standard solution
Sample solution: 1 mg/ml, of Enalapril Maleate in Diluent Sonicate to dissolve an needed.
Chromatographic system
(See Shamayanti Sotem Sul)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 5-μm packing L7
Temperatures
Autocampler: 20°
Colume 70°
Flew rate: 1.5 mL/min
Injection volume: 50 μL
System suitability
Samples: System suitability solution, Standard solution, and Senalivity anlution
No-See Table for the relative retention times
Sultability requirements
Resolution: LT 1.0 between monsipil related compound and enalapril System suttatality anlation
Tailing factor: NMT 2.0 Standant sution
Relative standard deviation: NMT 5.0%, Standard soun
Signal-to-noise ratio: NLT 10. Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of of Enalapril Maleate taken:
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of Enalapril Maleate from the Sample solution
rS = peak response of Enalapril Maleate from the Standard solution
CS = concentration of USP Enalapril Maleate RS in the Standard solution (mg/ml)
CU = concentration of Enalapril Maleate in the Sample solution (mg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Maleic acida | 0.13 | - | - |
| Enalaprilatb | 0.55 | 1.41 | 0.3 |
| Moexipril related compound F | 0.93 | 0.85 | 0.3 |
| Enalapril | 1.00 | - | - |
| Enalapril cyclohexyl analogc | 1.33 | 0.45 | 0.3 |
| Enalapril related compound Dd | 1.48 | 1.27 | 0.3 |
| Any unspecied impurity | - | 1.00 | 0.10 |
| Total impurities | - | - | 2.0 |
a This peak is due to the counterion and is not to be reported or included in the total impurities.
b 1-[N-[(S)-1-Carboxy-3-phenylpropyl]-l-alanyl]-l-proline.
c 1-{N-[(S)-1-Ethoxycarbonyl-3-cyclohexylpropyl]-l-alanyl}-l-proline.
d (S)-Ethyl 2-[(3S,8aS)-3-methyl-1,4-dioxohexahydropyrrolo[1,2-α]pyrazin-2(1H)-yl]-4-phenylbutanoate.
5 SPECIFIC TESTS
Optical Rotation (781S), Procedures, Specific Rotation
Sample solution: 10 mg/mL in methanol
Acceptance criteria: −41.0° to −43.5°
Loss on Drying (731)
Analysis: Dry under vacuum at a pressure not exceeding 5 mm of mercury at 60° for 2 h.
Acceptance criteria: NMT 1.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, and store at controlled room temperature.


