Edrophonium Chloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
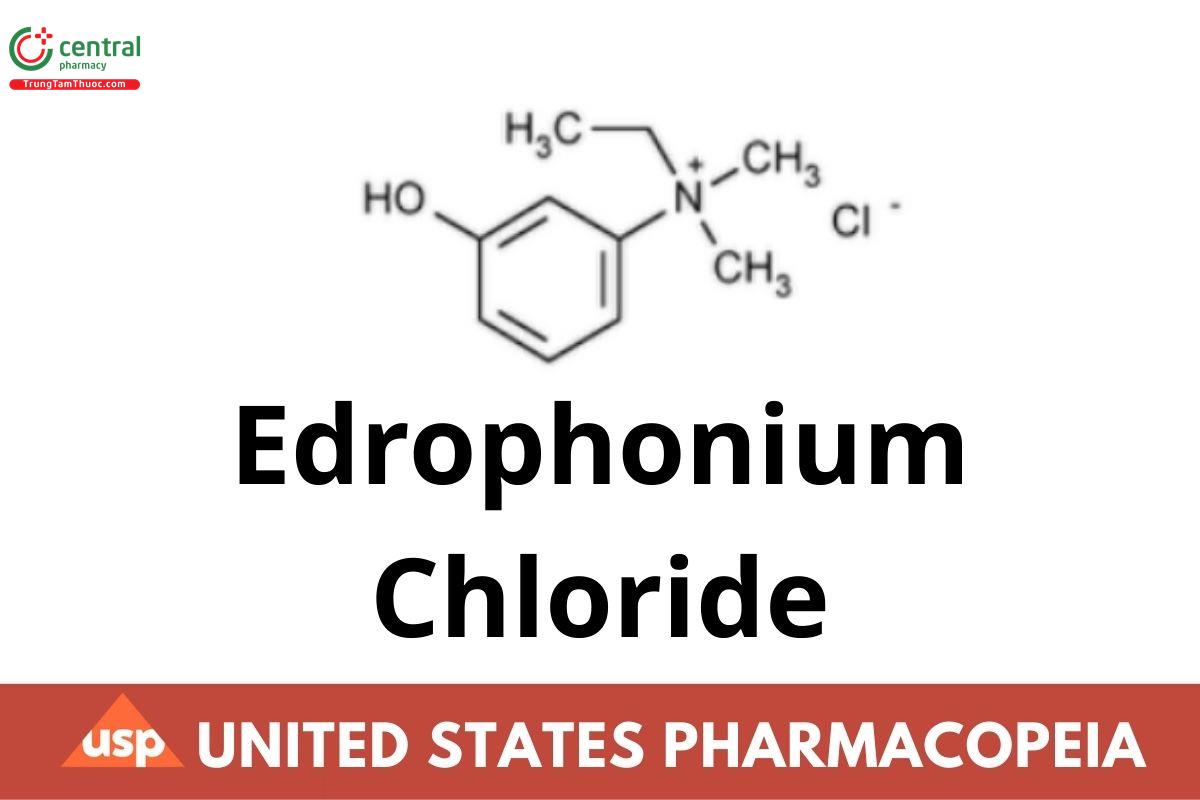
Edrophonium Chloride contains not less than 98.0 percent and not more than 100.5 percent of C10H16ClNO, calculated on the dried basis.
1 Packaging and storage
—Preserve in well-closed containers.
USP Reference standards 〈11〉—
USP Edrophonium Chloride RS
2 Identification—
Change to read:
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020).
Change to read:
B: Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020) —
Solution: 50 μg per mL.
Medium: 0.1 N hydrochloric acid.
Absorptivities at 273 nm, calculated on the dried basis, do not differ by more than 2.0%.
C: To 10 mL of a solution (1 in 10) add 1 drop of ferric chloride TS: a violet-blue color is produced.
D: It responds to the tests for Chloride 〈191〉.
Melting range 〈741〉: between 165° and 170°, with decomposition.
pH 〈791〉: between 4.0 and 5.0, in a solution (1 in 10).
Loss on drying 〈731〉—Dry it in a suitable vacuum drying tube, using phosphorus pentoxide as the desiccant, for 3 hours: it loses not more than 0.5% of its weight.
Residue on ignition 〈281〉: not more than 0.1%.
Limit of dimethylaminophenol—Dissolve about 500 mg, accurately weighed, in 5 mL of water in a separator. Add 5 mL of pH 8.0 phosphate buffer (see Buffer Solutions in the section Reagents, Indicators, and Solutions), and shake. Extract with five 20-mL portions of chloroform, pooling the extracts in a 100-mL volumetric flask, and add chloroform to volume. The absorbance of this solution, determined in a 1-cm cell at 252 nm, with a suitable spectrophotometer, is not more than that of a 1 in 200,000 solution of dimethylaminophenol in chloroform, similarly measured (0.1%).
3 Assay
—Transfer about 175 mg of Edrophonium Chloride, accurately weighed, to a suitable flask, and dissolve in about 20 mL of glacial acetic acid. Add 5 mL of mercuric acetate TS, then add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 20.17 mg of C10H16ClNO.


