Doxylamine Succinate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
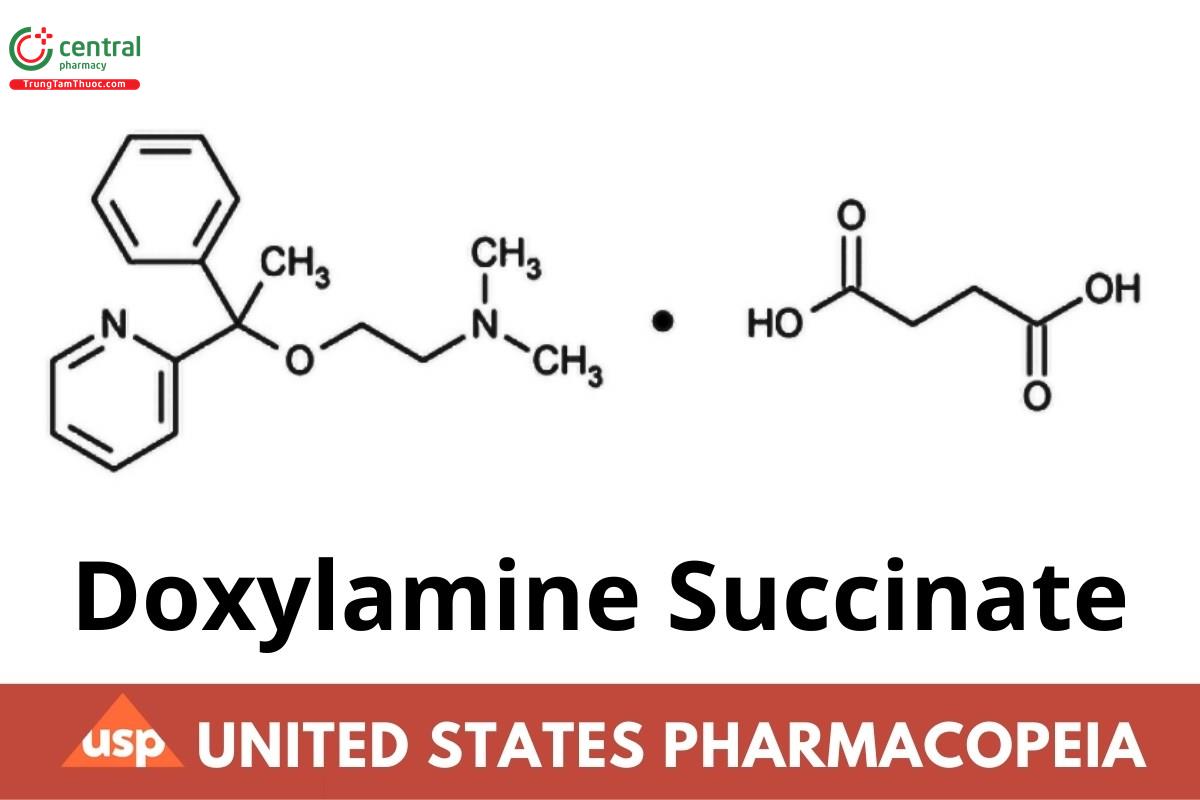
Ethanamine, N,N-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]-, butanedioate (1:1);
2-[α-[2-(Dimethylamino)ethoxy]-α-methylbenzyl]pyridine succinate (1:1);
N,N-Dimethyl-2-[1-phenyl-1-(pyridin-2-yl)ethoxy]ethan-1-amine succinate CAS RN®: 562-10-7.
1 DEFINITION
Change to read:
Doxylamine Succinate contains NLT 98.0% and NMT 102.0% (USP 1-May-2019) of doxylamine succinate (C17H22N2O · C4H6O4), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
ASPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy 1970 (CN 1--2020)
Analytical wavelength: 262 nm
Sample solution: 20 µg/mL of Doxylamine Succinate in 0.1 N hydrochloric acid
Acceptance criteria: Absorptivities, calculated on the dried basis, do not differ by more than 3.0%
Delete the following:
B. IDENTIFICATION ORGANIC NITROGENOUS BASES (181): It meets the requirements (USP 1-May-2019)
Add the following:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay (USP 1-May-2019)
C.
Sample solution: Dissolve 500 mg of Doxylamine Succinate in 5 ml of water, and add a slight excess of 6 N ammonium hydroxide. Extract the liberated doxylamine with several portions of ether, discard the ether extracts, and evaporate the aqueous solution on a steam bath to dryness. Add 2 mL of 3 N hydrochloric acid, and again evaporate on the steam bath to dryness. Cool, add about 10 mL of ether, allow to stand for a few minutes, and decant the clear supernatant. Evaporate the ether solution to dryness, and dry the residue at 105° for 30 min.
Acceptance criteria: The succinic acid melts between 184" and 188", as indicated for Class I under Melting Range or Temperature (741), Procedures
3 ASSAY
Change to read:
PROCEDURE
Solution A: 50 mM ammonium acetate in water, adjusted with glacial acetic acid to a pH of 4.0
Solution B: Acetonitrile
Mobile phase: See Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 2 | 90 | 10 |
| 15 | 40 | 60 |
| 20 | 40 | 60 |
| 21 | 90 | 10 |
| 25 | 90 | 10 |
Diluent: Solution A and Solition (9010)
Standard solution: 0.1 mg/ml of USP Dondamine Succinate RS Σλλητ
Sample solution: 0.1 mgnnt of Doxylamine Suconate in Diluent
Chromatographic system
(See Chromatogracity 1021 System Stay)
Mode: LC
Detector: UV 252.nm
Columns: 4.5mx15cm, 8um packing
Column temperature: 30
Flow rate: 1 mL/mem
Injection volume: 20
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2:0
Relative standard deviation: NMT 0,73%
AL
Analysis
Samples: Standards and Sample solution
Calculate the percentage of doxylamine succinate (C17H22N2O · C4H6O4) inthe portion of Dorylamine Suconate taken:
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of doxylamine from the Sample solution
rS = peak response of doxylamine from the Standard solution
CS = concentration of USP Doxylamine Succinate RS in the Standard solution (mg/mL)
CU = = nominal concentration of doxylamine succinate in the Sample solution (mg/ml)
Acceptance criteria: 90.05-1020 on the dried basis.
4 IMPURSTIES
Residue on Ignition (281): NMT 0.1%
Delete the following:
VILAFEL RILATED COMPONES
Sample solution: Dissolve 650 mg in 20 ml of 0.1 N hydroctooricand in separator. Rende the solution alkaline with 2.5 N sodium hydroxide, and immediately extract with four 25-ml, portions of ether, filtering each extract through an ether saturated pledget of cotton. Evaporate the combined ether extracts on a water bath with the aid of a current of ale to dryness at a temperature net excserting 50" and dissolve the residue in 5 mL of alcohol.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: DC
Detector: Fissmelonization
Column: 4-mm x 2m glass column containing 5% packing 1210 and 5% pecking 912 on 60- to BUmesh 516
Temperatures
Colume: 212°
Injection port: 250°
Detector block: 250°
Carrier gas: Dry helium
Injection volume: 1μL
Acceptance criteria: NMT 2.00%, the total relative arms of all extraneous peaks (except that of the solvent peak), ane NMT 1.0, the relative area of any individual extraneous peak
Add the following:
Solution A, Solution B, Mobile phase, Diluent, and Chromatographic systent Proceed as directed in the Assay
Standard solution: 0.001 mg/ml each of oxviamine Succinate RS and Carbinoxamine Related Compound CRG in Diuent
Sample solution: 10 mg/ml, of Doxylamine Succinate in Diluent
System suitability
Sample: Standard solution
Nom-See Table 2 for relative retention times
Suitability requirements
Resolution: NUT 2 between doxylammine and carbinoxamine related compound C
Relative standard deviation: NMT 5.0% for the doxylamine and carbinoxamine related compound C peaks
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of carbinoxamine related compound C in the portion of Doxylamine Suconate takerc
Result = (rU/(rS) × (CS /CU) x 100
rU = peak response of carbonoxamine related compound C from the Sample solution
rS = peak response of cartenoxamine related compound C from the Standard solution
CS = concentration of USP Carbinoxamine Related Compound C RS in the Standard solution (mg/mL)
CU = concentration of Doxylamine Succinate in the Sample solution (mg/ml)
Calculate the percentage of each specified and any unspecified impurity in the portion of Doxylamine Succinate taken:
Result = (rU/(rS) × (CS /CU) x (1/F) x 100
rU = peak response of each specified and any unspecified impunity from the Sample solution
rS = peak response of coxylamine from the Standard solution
CS = concentration of USP Doxylamine Succinate RS in the Standard solution (mg/mL)
CU = concentration of Doxylamine Succinate in the Sample solution (mg/mL)
F = relative response factor of each specified and any unspecited impurity (see Table 2)
Acceptance criteria: See Table 2. The reporting threshold is 0.05%
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Doxylamine pyridinyl N-oxidea | 0.64 | 2.1 | 0.10 |
| Doxylamine dioxideb | 0.70 | 1.8 | 0.10 |
| Doxylamine pyridine-4-yl isomerc | 0.81 | 1.0 | 0.15 |
| Carbinoxamine related compound C | 0.95 | - | 0.15 |
| Doxylamine | 1.0 | - | - |
| Doxylamine ethylamine N-oxided | 1.06 | 1.0 | 0.10 |
| Doxylamine alcohole | 1.30 | 1.8 | 0.15 |
| 2-Benzoylpyridinef | 1.44 | 5.8 | 0.15 |
| Any unspecified impurities | - | 1.0 | 0.10 |
| Total impurities | - | - | 1.0 |
a 2-[1-(2-(Dimethylamino)ethoxy)-1-phenylethyl]pyridine 1-oxide.
b N,N-Dimethyl-2-(1-(1-oxidopyridin-2-yl)-1-phenylethoxy)ethan-1-amine oxide
c CN,N-Dimethyl-2-11-phenyl-1-pyridin-4-yl)ethoxyjethan-1-amine
d Process related impurity. Do not include in calculation of total degradation products.
e NN-Dimethyl-2-11-phenyl-1-(pyridin-2-yl)ethoxylethan-1-amine oxide
f 1-Phenyl-1-(pyridin-2-yl)ethan-1-ol.
g Phenyl (pyridin-2-yl)methanone.
5 SPECIFIC TESTS
Delete the following:
Melting Range or Temperature, Class I (741): 103°–108°, the range between the beginning and end of melting does not exceed 3°.
Loss on Drying (731)
Analysis: Dry under vacuum over phosphorus pentoxide for 5 h.
Acceptance criteria: NMT 0.5%
ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers.


