Doxycycline Tablets
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
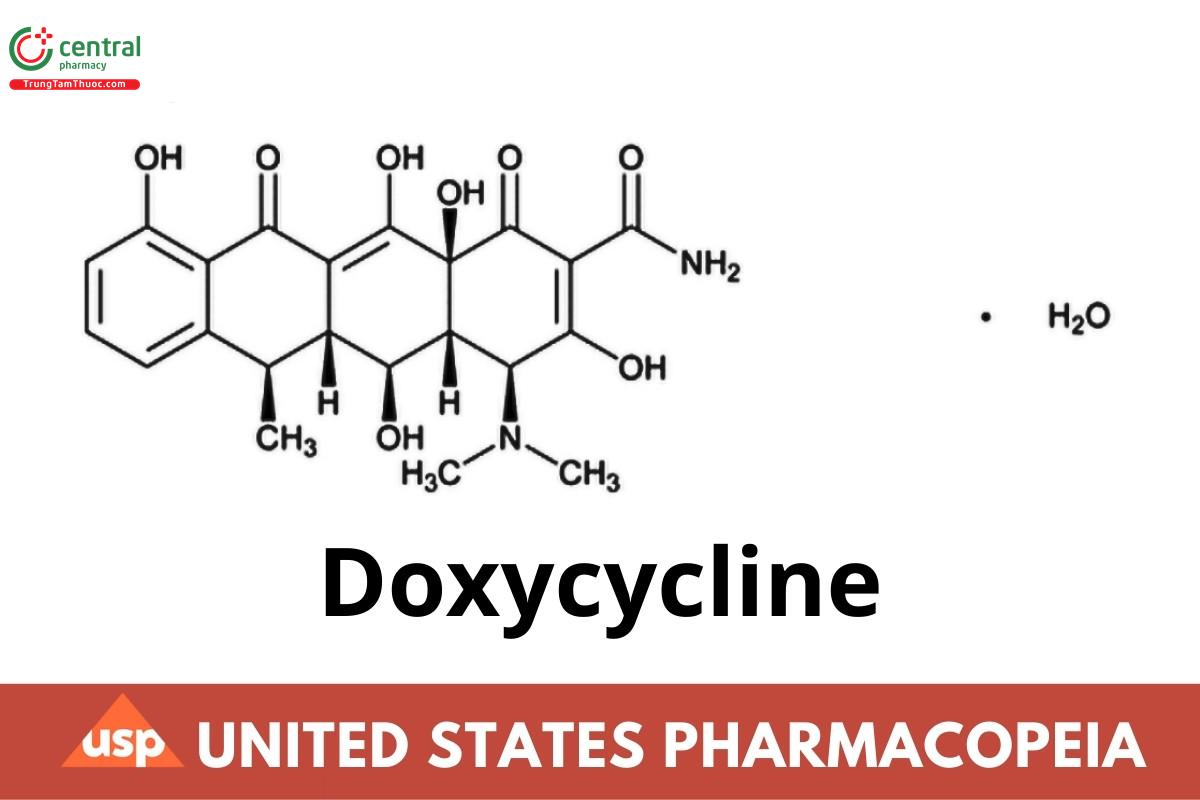
1 DEFINITION
Doxycycline Tablets contain NLT 90.0% and NMT 120.0% of the labeled amount of doxycycline(C22H24N2O8).
2 IDENTIFICATION
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. The UV spectrum of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Protect solutions containing doxycycline from light.
Solution A: Transfer 3.1 g of monobasic potassium phosphate, 0.5 g of edetate disodium, and 0.5 mL of triethylamine to a 1000-ml volumetric flask. Add about 850 ml of water and mix. Dilute with water to volume and adjust with 1 N sodium hydroxide to a pH of 8.5± 0.1.
Solution B: Methanol
Mobile phase: See Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
| 0.0 | 90 | 10 |
| 2.0 | 90 | 10 |
| 4.0 | 60 | 40 |
| 6.0 | 90 | 10 |
| 9.0 | 90 | 10 |
Diluent: 0.01 N hydrochloric acid
Standard solution: 0.12 mg/ml, of USP Doxycycline Hyclate RS in Diluent. Sonicate as needed to dissolve.
Sample solution: Nominally 0.1 mg/mL of doxycycline from NLT 20 Tablets prepared as follows: Transfer a suitable portion of finely powdered Tablets to a suitable volumetric flask. Add 50% of the final volume of Diluent, dissolve, dilute with Diluent to volume, and mix well.
Centrifuge a portion of the solution and use the supernatant. [Note-The use of a centrifuge speed at 3,000 rpm for 10 min may be suitable.]
Chromatographic system
(See Chromatography (621), System Suitability)
Mode: LC
Detector: UV 270 nm. For Identification B, a diode array detector may be used in the wavelength range of 200-400 nm.
Column: 2.1-mm x 5-cm, 1.7-um packing 12 (Νοτε-A 1.7-um guard column with packing 17 was used during method validation.J
Column temperature: 60"
Flow rate: 0.6 mL/min
Injection volume: 5 µl
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of doxycycline(C22H24N2O8) in the portion of Tablets taken:
Result = (rU/(rS) × (CS /CU) × P x F x 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Doxycycline Hyclate RS, in the Stardard solution (mg/ml)
CU = nomina concentration ofof Doxycycline Hyclate in the Sample sulation (mg/ml)
P = potency of methacycline in USP Methacycline Hydrochloride RS (μg/mL)
F = conversion factor, 0.001 mg/µg
Acceptance criteria: 90.0%-120.0%
4 PERFORMANCE TESTS
DISSOLATION (711)
Test 1
Medium: 0.01 N hydrochloric acid; 900 mL
Apparatus 2: 75 rpm
Time: 60 min
Standard solution: 0.01 mg/mL of doxycycline from USP Doxycycline Hyclate RS in Medium
Sample solution: Pass a portion of the solution under test through a suitable filter. Dilute a portion of the filtrate with Medium to a concentration that is similar to that of the Standard solution.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy (857))
Mode: LIV
Analytical wavelength: 268 nm
Cell: 1 cm
Blanc Medium
Analysis
Samples: Standard solution and Sample solution
Determine the percentage of the labeled amount of doxycycline (C22H24N2O8) dissolved:
Result = (AU/AS) x (CS/L) x V x P x 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of doxycycline in the Standard solution (mg/mL)
L = label claim (mg/Tablet)
V = volume of Medium, 900 mL
P = potency of doxycycline in USP Doxycycline Hyclate RS (µg/mg)
Tolerances: NLT 85% (Q) of the labeled amount of doxycycline (C22H24N2O8) is dissolved.
Test 2: if the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Protect solutions containing doxycycline from light.
Medium: 0.01 N hydrochlonc acid 900 ml
Apparatus 2: 75 rpm
Time: 15 min
Standard solution: 0.01 mg/mL of doxycycline from USP Doxycycline Hyclate RS in Medium
Sample solution: Pass a portion of the solution under test through a suitable filter. Dilute a portion of the filtrate with Medium to a concentration that is similar to that of the Standard solution
Instrumental conditions
(See Ultraviolet-Visible Spectroscолк (857))
Mode: UV
Analytical wavelength: 268 nm
Cell: 1 cm
Blank: Medium
Analysis
Samples: Standard solution and Sample solution
Determine the percentage of the labeled amount of doxycycline (C22H24N2O8) dissolved:
Result = (AU/AS) x (CS/L) × D × V × P × F × 100
AU = absorbance of the Sample solution
AS = absorbance of the Standard solution
CS = concentration of doxycycline in the Standard solution (mg/mL)
L = label claim (mg/Tablet)
D = dilution factor for the Sample solution, if applicable
V = volume of Medium, 900 mL
P = potency of doxycycline in USP Doxycycline Hyclate RS (µg/mg)
F = conversion factor, 0.001 mg/µg
Tolerances: NLT 80% (0) of the labeled amount of doxycycline(C22H24N2O8) is dissolved.
UNFORMITY OF DOSAGE UMT (905): Meet the requirements
5 IMPURITIES
ORGANIC IMPURITES
Protect solutions containing doxycycline from light.
Mobile phase, Diluent, and Chromatographic system: Proceed as directed in the Assay
System suitability stock solution 1:1 mg/mL each of USP Doxicycline Related Compound A RS and USP Methacycline Hydrochloride RS in Diluent
System suitability stock solution 2: 1.2 mg/mL of USP Doxycycline Hyclate RS in Diluent
System suitability solution: Transfer 5 ml of System suitability stock solution 2 to a 25-ml volumetric flask, heat on a steam bath for 60 min, and evaporate to dryness on a hot plate, taking care not to char the residue. Dissolve the residue in Diluent, add 0.5 mL of System suitability stock solution 7, and dilute with Diluent to volume Pass the solution through a suitable fitter and use the filtrate. This solution contains a mixture of 4-epidoxycycline, doxycycline related compound A, methacycline, and doxycycline (Nore The solution is stable up to 14 days when stored in a refrigerator at 2"-8"]
Standard solution: 7.0 µg/mL of UP Doxycycline Hyclate RS in Diluent
Sample solution: Nominally 2.0 mg/ml. of doxycycline from NLT 20 Tablets prepared as follows. Transfer a suitable portion of finely powdered Tablets to a suitable volumetric flask. Add 50% of the final volume of Diluent, dissolve, dilute with Diluent to volume, and mix well. Centrifuge a portion of the solution and use the supernatant. (Non-The use of a centrifuge speed at 3.000 rpm for 10 min may be suitable]
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 1.5 between methacycline and 4-epidoxycycline; NLT 1.5 between 4-epidoxycycline and doxycycline related compound A NLT 2.0 between doxycycline related compound A and doxycycline, System suitability solution
Relative standard deviation: NMT 5.0% for the doxycycline peak, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tablets taken:
Result = (rU/(rS) × (CS /CU) × P x F x 100
rU = peak response of each impurity from the Sample solution
rS = peak response of doxycycline from the Standard solution
CS = concentration of USP Doxycycline Hyclate RS in the Standard solution (mg/mL)
CU = nominal concentration of doxycycline in the Sample solution (mg/mL)
P = potency of doxycycline in USP Methacycline Hydrochloride RS (µg/mg)
F = conversion factor, 0.001 mg/µg
Acceptance criteria: See Table 2 Disregard peaks less than 0.1%
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Methacyclinea,b | 0.64 | - |
| 4-Epidoxycyclinec | 0.79 | 1.5 |
| Doxycycline related compound A (6-epidoxycycline)b,d | 0.88 | - |
| Doxycycline | 1.0 | - |
| Any individual unspecified impurity | - | 0.3 |
| Total impurities | - | 2.5 |
a (4S,4aR,55,5aR, 12aS)-4-(Dimethylamino)-1,4,4,5,58,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacenecarboxamide.
b (4R,4aR,55,5aR,6,12aS)-4-(Dimethylamino)-1,4,4,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide.
c(4R,4aR,5S,5aR,6R,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2- naphthacenecarboxamide.
d (4S,4aR,5S,5aR,6S,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2 naphthacenecarboxamide.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store at controlled room temperature.
Labeling: When more than one Dissolution test is given, the labeling states the test used only if Test 1 is not used.


