Doxycycline
If you find any inaccurate information, please let us know by providing your feedback here
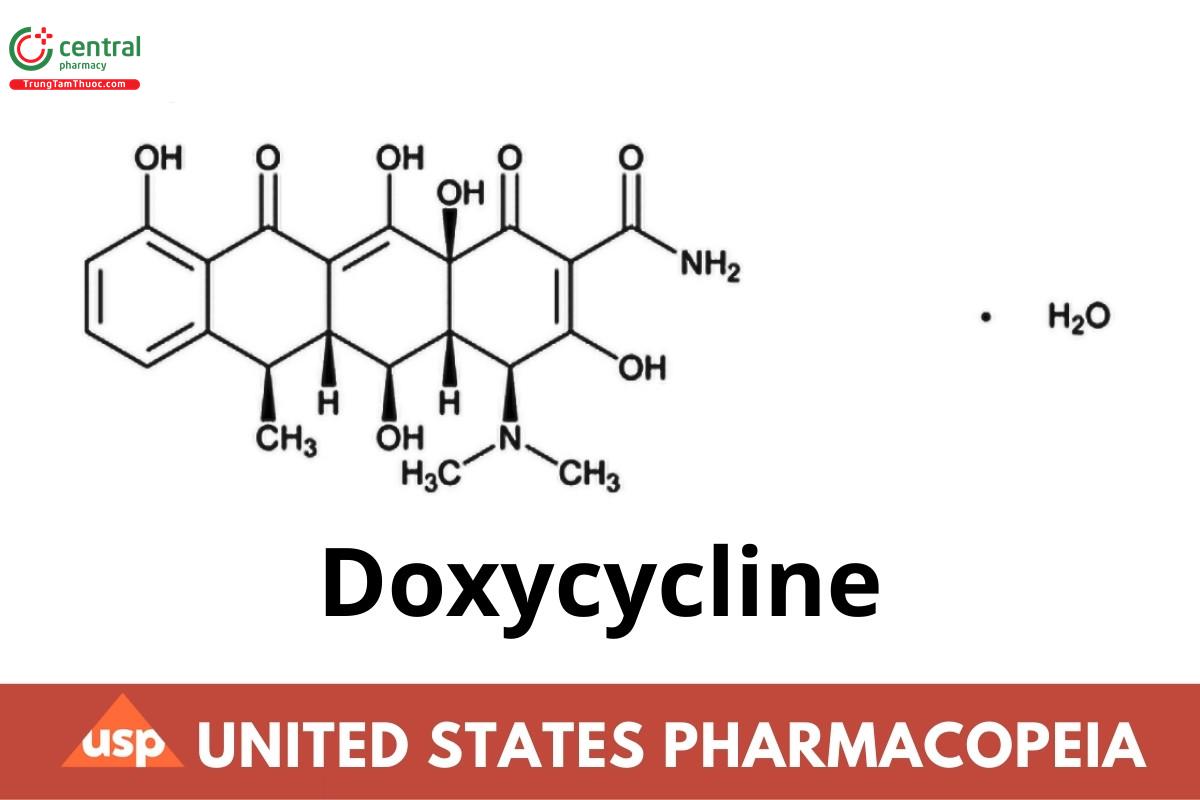
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, [4S-(4α,4aα,5α,5aα,6α,12aα)]-, monohydrate;
(4S,4aR,5S,5aR,6R,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrate CAS RN®: 17086-28-1; UNII: N12000U13O.
Anhydrous CAS RN®: 564-25-0; UNII: 334895S862.
1 DEFINITION
Doxycycline has a potency equivalent to NLT 880 µg/mg and NMT 980 µg/mg of doxycycline (C22H24N2O8).
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay
3 ASSAY
PROCEDURE
Protect solutions containing doxycycline from light
Solution A: Transfer 3.1 g of monobasic potassium phosphate and 0.5 g of of edetate disodium to a 1000-mL volumetric flask. Add about 850 ml of water and 0.5 ml of triethylamine, and mix. Dilute with water to volume and adjust with 1 N sodium hydroxide to a pH of 8.5 plus/minus 0.1 .
Solution B: Methanol
Mobile phase: See Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
| 0.0 | 90 | 10 |
| 2.0 | 90 | 10 |
| 6.0 | 85 | 15 |
| 8.0 | 60 | 40 |
| 8.1 | 90 | 10 |
| 10.0 | 90 | 10 |
Diluent: 0.01 N hydrochloric acid
Standard solution: 0.1 mg/ml. of USP Doxycycline Monohydrate RS in Diluent. Sonicate as needed to dissolve.
Sample solution: 0.1 mg/mL of Doxycycline in Diluent. Sonicate as needed to dissolve.
Chromatographic system
(See Chromatography (621), System Suitability.)
USP-NF Doxycycline
ps/PuPigtamthuoc.com/
Mode: LC
Detector: UV 270 nm
Column: 2.1-mm x 5-cm, 1.7-µm packing LZ
Column temperature: 60°
Flow rate: 0.6 mL/min
Injection volume: 5 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the quantity of doxycycline (C22H24N2O8) 8 in µg/mg, in the portion of Doxycycline taken:
Result = (rU/(rS) × (CS /CU) × P
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Doxycycline Monohydrate RS, in the Stardard solution (mg/ml)
CU = nominal concentration of doxycycline in the Sample solation (mg/ml)
P = potency of doxycycline in USP Doxycycline Monohydrate RS (µg/mg)
4 IMPURITIES
Change to read:
ORGANIC IMPURITIES
Mobile phase, Diluent, and Chromatographic system: Proceed as directed in the Assay.
System suitability stock solution 1: 1 mg/ml each of USP Methacycline Hydrochloride RS and USP Doxycycline Related Compound A RS in Diluent
System suitability stock solution 2: 1.2 mg/mL of USP Doxycycline Hyclate RS in Diluent
System suitability solution: Transfer 5 mL of System suitability stock solution 2 to a 25-ml volumetric flask, heat on a steam bath for 60 min, and evaporate to dryness on a hot plate, taking care not to char the residue. Dissolve the residue in Diluent, add 0.5 ml. of System suitability stock solution 1, and dilute with Diluent to volume Pass the solution through a suitable filter and use the filtrate. This solution contains a mixture of 4-epidoxycycline, methacycline, doxycycline related compound A, and doxycycline. When stored in a refrigerator, this solution may be used for 14 days.
Sensitivity solution: 0.001 mg/mL of USP Doxycycline Monohydrate RS in Diluent
Standard solution: 0.002 mg/ml, each of USP Doxycycline Monohydrate RS and USP Methacycline Hydrochloride RS in Diluent
Sample solution: 2 mg/mL of Doxycycline in Diluent. Sonicate as needed to dissolve.
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
Suitability requirements
Resolution: NLT 1.5 between methacycline and 4-epidoxycycline, NLT 1:5 between 4-epidoxycycline and doxycycline related compound A
NLT 2.0 between doxycycline related compound A and doxycycline, System suitability solution
Relative standard deviation: NMT 5.0% each for the doxycycline and methacycline peaks, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of methacycline in the portion of Doxycycline taken:
Result = (rU/(rS) × (CS /CU) × P x F x 100
rU = peak response of methacycline from the Sample solution
rS = peak response of methacycline from the Standard solution
CS = concentration of USP Methacycline Hydrochloride RS, in the Stardard solution (mg/ml)
CU = concentration of doxycycline in the Sample solation (mg/ml)
P = potency of doxycycline in USP Methacycline Hydrochloride RS (µg/mg)
F = conversion factor, 0.001 mg/µg
Calculate the percentage of 4-epidoxycycline, doxycycline related compound A, doxycycline related compound F, and any individual unspecied impurity in the portion of Doxycycline taken:
Result = (rU/(rS) × (CS /CU) × P x F1/F2 x 100
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Methacycline | 0.51 | 1.0 | 2.0 |
| 4-Epidoxycyclinea | 0.60 | 1.0 | 0.5 |
| Doxycycline related compound A (6-epidoxycycline)b,d | 0.72 | 0.67 | 2.0 |
| Doxycycline | 1.0 | - | - |
| Doxycycline related compound Fb | 1.20 | 0.66 | 1.0 |
| Any individual unspecified impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 2.5 |
a(4R,4aR,5S,5aR,6R,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-
naphthacenecarboxamide. Main degradation product.
b (4S,4aR,5S,5aR,6R,12aS)-2-Acetyl-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-4a,5a,6,12a-tetrahydrotetracene-1,11(4H,5H)-
dione.
5 SPECIFIC TESTS
CRYSTALLINITY (695): Meets the requirements.
PH (791)
Sample solution: An aqueous suspension containing 10 mg/ml.
Acceptance criteria: 5.0-6.5
WATER DETERMINATION (921), Method: 3.6%-4.6%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.


