Doxorubicin Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
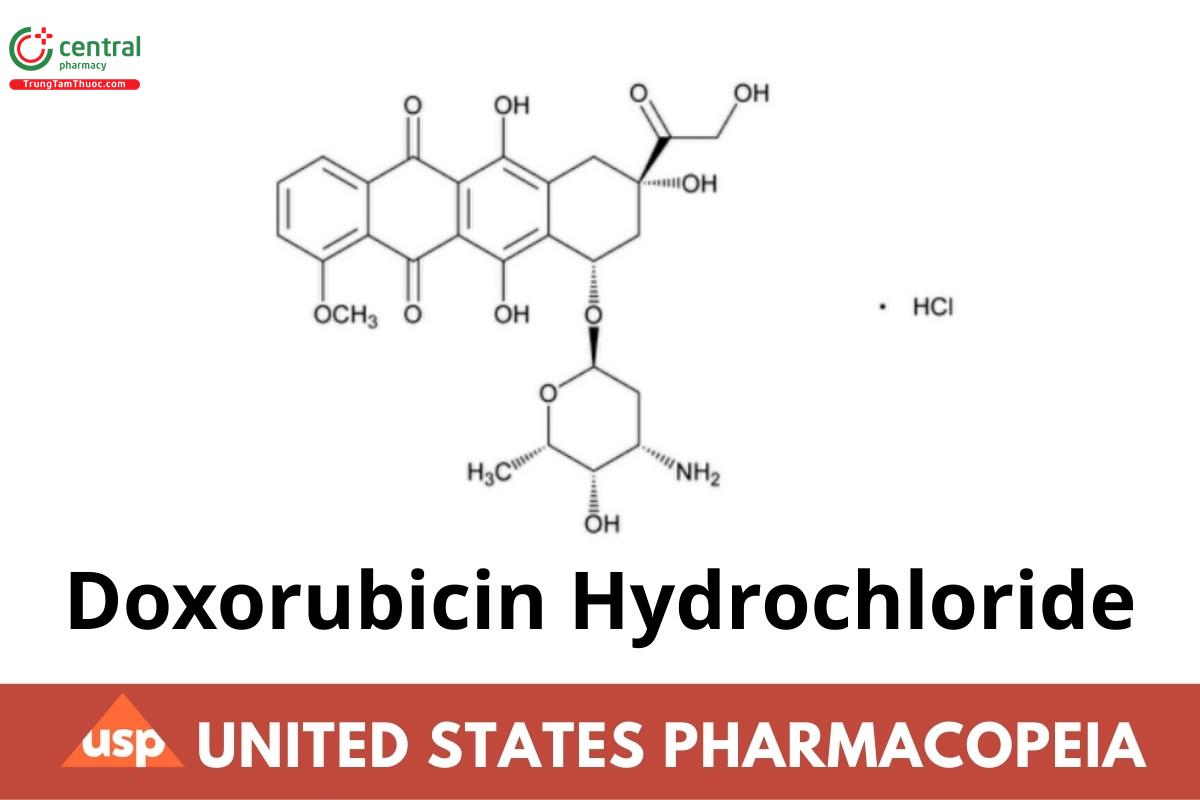
5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-a--lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxylacetyl) methoxy-, hydrochloride (8S-cis)-;
(8S,10S)-10-[(3-Amino-2,3,6-trideoxy-a--lyxo-hexopyranosyl)oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-
naphthacenedione hydrochloride CAS RN®: 25316-40-9; UNII: 82F2G7BL4E.
1 DEFINITION
Doxorubicin Hydrochloride contains NLT 98.0% and NMT 102.0% of doxorubicin hydrochloride (C27H29NO11· HCl), calculated on the anhydrous, solvent-free basis.
[CAUTION-Great care should be taken to prevent inhaling particles of doxorubicin hydrochloride and exposing the skin to it.]
2 IDENTIFICATION
A. The retention time of the doxorubicin peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
C. IDENTIFICATION TESTS GENERAL, Chloride (191)
3 ASSAY
Change to read:
PROCEDURE
Solution A: 0.1% Trifluoroacetic acid prepared by transferring 1.0 mL of trifluoroacetic acid to 1 L of water
Solution B: Acetonitrile, methanol, and trifluoroacetic acid (800:200:1)
Mobile phase: See Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 15 | 25 | 75 |
| 16 | 25 | 75 |
| 16.1 | 90 | 10 |
| 18 | 90 | 10 |
Diluent: Solution A and Solution B (50:50)
[NOTE-Protect solutions containing doxorubicin from light.]
System suitability solution: 0.1 mg/ml, each of USP Doxorubicin Hydrochloride RS and USP Epirubicin Hydrochloride RSA (ERR 1-May-2024) In
Diluent
Standard solution: 0.1 mg/ml, of SP Domubicin Hydrochloride RS in Dient
Sample solution: 0.1 mg/ml, of Doxorubicin Hydrachlonde in Cluent
Chromatographic system
(See Chromatopanky (1211. Smm Sumalday)
Mode: LC
Detector: UV 254 m
Column: 2.1mm x 10cm, 1.7-um packing Li
Temperatures
Column: 35
Autosampler: 4
Flow rate: 0.5 ml/min
Injection volume: 2 με
System suitability
Samples: System sutatility solution and Standard solution
[Nom-The relative retention times for doxorubicin and epirubicin are 1.0 and 1.05, respectively]
Suitability requirements
Resolution: NLT 1.5 between doxorubicin and eprubicin, System surhabdity solution
Tailing factor: 0.8-1.5. Standard solution
Relative standard deviation: NMT 73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of doxorubicin hydrochloride (CHNO, HCI) in the portion of Doxorubicin Hydrochloride taken:
Result = (ru/rs) × (Cs/Cu) × P × F × 100
ru = peak response of doxorubicin from the Sample solution
rs = peak response of doxorubicin from the Standard solution
Cs = concentration of USP Dosunibicin Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Doxorubicin Hydrochloride in the Sample solution (mg/ml)
P = potency of doxorubicin hydrochloride in USP Doxorubicin Hydrochlonde RS (µg/mg)
F = conversion factor, 0.001 mg/ول
Acceptance criteria: 98.0%-102.0% on the anhydrous, sofvent-free basis
4 IMPURITIES
Mobile phase, Diluent, System suitability solution, and Chromatographic system: Proceed as directed in the Assay.
Nore-Protect solutions containing doxorubicin from light.
Standard solution: 0.002 mg/ml, each of bicin Hydrochloride RS USP Respubicinome RS, JUSP Daunorubicon Hydrochinnde RS and USP Daunorubicinone RS in
Sample solution: 0.4 mg/mL of Doxorubicin Hydrochloride in Diluent
System suitability
Samples: System suitability solution and Standard solution
[Non-See Table 2 for the relative retention times.]
Suitability requirements
Resolution: NLT 1.5 between doxorubicin and spirubicin, System suhability solution
Relative standard deviation: NMT 5.0 Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of doxorubicinone in the portion of Doxorubicin Hydrochloride taken
Result = (ru/rs) × (Cs/Cu) × P × F × 100
ru = peak response of doxorubicinone from the Sample solution
rs = peak response of doxorubicinome from the Standard solution
Cs = Cconcentration of USP Doxorubicinone RS in the Standard solution (mg/m)
Cu = concentration of Doxorubicin Hydrochloride in the Sample solution (mg/ml)
P = potency of doxorubicinone in the USP Doxorubicinone RS (mg/mg)
Calculate the permentage of daunorubicinione in the portion of Doxorubicin Hydrochloride taken
Result = (ru/rs) × (Cs/Cu) × P × F × 100
ru = peak response of daunorubicinone from the Sample solution
rs = peak response of dounorubicinone from the Standard solution
Cs = concentration of USP Daunorubicinone RS in the Standard solution (mg/ml)
Cu = concentration of Doxorubicin Hydrochloride in the Sample solution (mg/mL)
P = potency of daunorubicinone in USP Daunorubicinone RS (mg/mg)
Calculate the percentage of daunonibicin in the portion of Doxorubicin Hydrochloride taken
Result = (ru/rs) × (Cs/Cu) × P × F × 100
ru = peak response of daunorubicin from the Sample solution
rs = peak response of daunarubicin from the Standard solution
Cs = concentration of USP Daunenidicin Hydrochloride RS in the Standard solution (mg/ml)
Cu = concentration of Doxorubicin Hydrochloride in the Sample solution (mg/ml)
P = potency of dautorubicin in USP Daunorubicin Hydrochloride RS (pg/mg)
F = convension factor, 0.001 mg/ug
Calculate the percentage of any individual unspecified impurity in the portion of Doxorubicin Hydrochloride taken
Result = (ru/rs) × (Cs/Cu) × P × F × 100
ru = peak response of any individual unspecified impurity from the Sample solution
rs = peak response of doxorubicin from the Standard solution
Cs = concentration of USP Doxorubicin Hydrochloride Rain the Standard solution (mg/mL)
Cu = concentration of Doxorubicin Hydrochloride in the Sample solution (mg/mL)
P = potency of doxorubicin hydrochloride in USP Duxordion Hutochloride RS5 (g/mg)
F = conversion factor 0.001 mg/g
Acceptance criteria: See Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Doxorubicin | 1.0 | - |
| Epirubicin | 1.05 | - |
| Doxorubicinone | 1.08 | 0.5 |
| Daunorubicin | 1.23 | 0.5 |
| Daunorubicnone | 1.35 | 0.5 |
| Any individual unspecified ampurity | - | 0.5 |
| Total impurities | - | 2.0 |
aFor resolution measurement only. Not to be reported not to be included in total impurities
b(88,105)-6,8,10,11-Tetrahydoxy-8-hydroxyacetyl-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione
c(82.105)-8-Acetyl-6,8,10,11-tetrahydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5, 12-dione
LANTIFACTURE AND ALCOHOL
Internal standard solution: 1 mg/ml of dioxane in water
Standard solution: 0.2 mg/ml. of 15P Acetone RS 0.3 mg/ml, of dehydrated alcohol in internal standard solution
Sample solution: 200 mg of Doxorubicin Hydrochloride in 30 ml (3.0 g) of internal standard solution
Chromatographic system
(See Chromatoglashy/621) Sutem Suitability)
Mode: GC
Column: 4mm 2m packed with 8-10% liquid phase 16 and 25% potassium hydroxide on 100-to 120 mesh support STA
Detector: Flame ionization
Column temperature: 60
Carrier gas: Helum
Nore-Adjust the column temperature and comer gas flow rate so that dioxane elutes in about 6 min
Injection volume: 1 L
Flow rabe: Adjust the column temperature and carrier gas flow rate so that dioxane elutes in about 6 min.
System suitability
Sample: Standard solution
Nore-The relative retention tames for acetone, alcohol, and dioxane are about 02,05, and 10, respectively
Suitability requirements
Resolution: NLT 2.0 between adjacent peaks
Tailing factor: NMT 1.5 for the alcohol pesk
Relative standard deviation: NMT 4.0% for the peak response ratios of acetone and alcohol to the internal standard
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage, by weight, of acetone and alcohol respectively, in the portion of Doxorubicar Hydrochloride taken
Result = (RU/RS) × (CA/CD) × (DU/WU) × 100
RU = peak response ratio of the analyte (acetone or alcohol) to dioxane from the Sample solution
RS = peak response ratio of the analyte (acartone or alcohol) to dioxane from the Standard solution
CA = concentration of the analyte (acetone or alcohol in the Standard solution (mg/ml)
CD = concentration of dioxane in the Standard solution (mg/mL)
DU = Weight of dioxane in the Sample solution (mg)
WU = Weight of Doxorubicin Hydrochloride in the Sample solution (mg)
Acceptance criteria
Acetone: NMT 0.5%
Total of soetone and alcohol: NMT 2.5%
5 SPECIFIC TESTS
COSTALNTY (095) Meets the requirements, except where it is labeled as amorphous, most particles do not exhibit birefringence and
extinction positions
Sample solution: 5 mg/mL
Acceptance criteria: 4.0-5.5
Ware Datos Method 11221 NMT 4.0%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STowce Preserve in tight containers, and store at controlled room temperature except where it is labeled as amorphous, in which case it should be stored in the freezer
LABLELING: The amorphous form is so labeled.


