Doxazosin Mesylate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
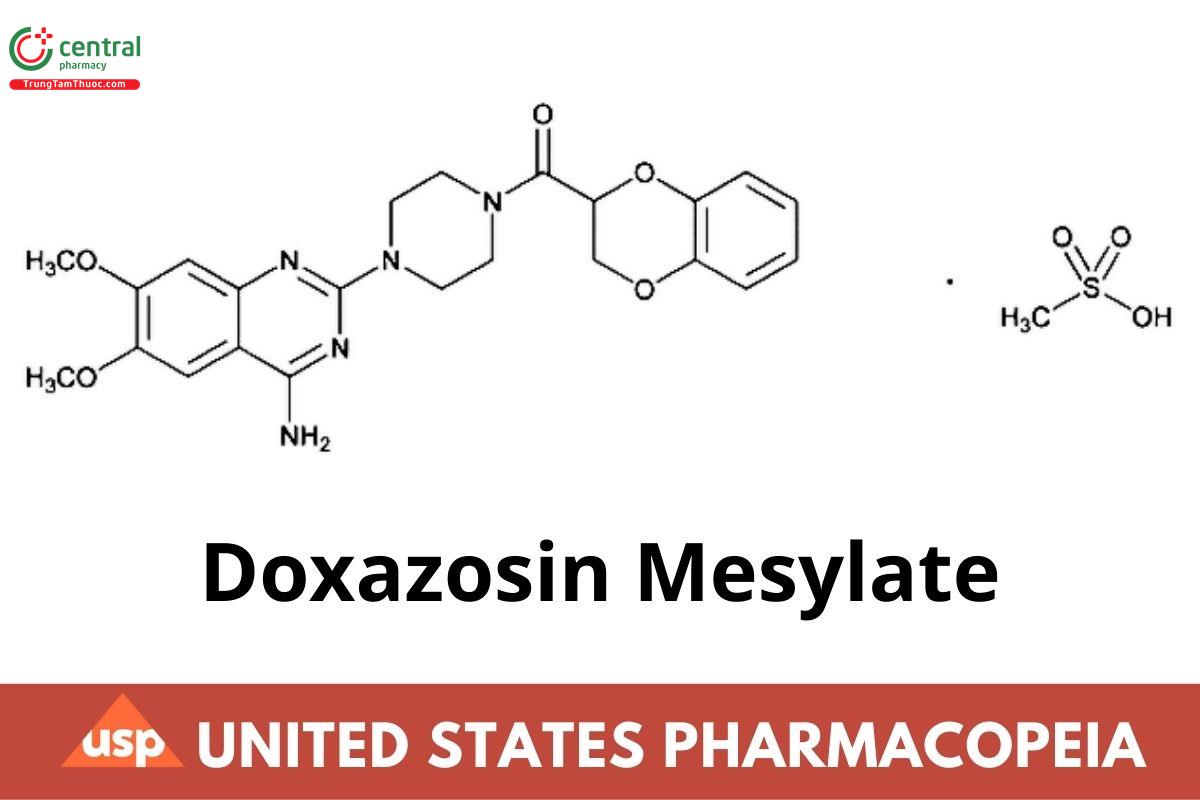
C23H25N5O5 · CH4O3S 547.58
Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(2,3-dihydro-1,4-benzodioxin-2-yl)carbonyl]-, monomethanesulfonate;1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl)piperazine monomethanesulfonate CAS RN: 77883-43-3; UNII:86P6PQK0MU.
1 DEFINITION
Doxazosin Mesylate contains NLT 98.0% and NMT 102.0% of doxazosin mesylate (C23H25N5O5 · CH4O3S), calculated on the dried basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Solution A: 50 mg/mL (w/v) of phosphoric acid in water
Solution B: Acetonitrile
Solution C: Water
Solution D: Mix 100 mL of Solution B and 2 g of phosphoric acid.
Mobile phase: See Table 1.
Table 1a
| Time (min) | Solution A (%) | Solution B (%) | Solution C (%) |
| 0 | 20 | 10 | 70 |
| 10 | 20 | 22 | 58 |
| 35 | 20 | 50 | 30 |
| 40 | 20 | 50 | 30 |
a Between sample injections, the system is re-equilibrated for at least 7 min or until a stable baseline is obtained, representing the starting composition.
System suitability solution: 12 µg/ml. of USP Doxazosin Related Compound A RS and 12 µg/mL of USP Doxazosin Related Compound B RS in a mixture of Solution C and Solution D (9:1), Initially add about 2.5 mL of Solution D and then add Solution D or Solution C to maintain a final composition of Solution C and Solution D in the ratio of 9:1. Sonicate briefly for complete dissolution.
Standard solution: 0.6 mg/mL of USP Doxazosin Mesylate RS in a mixture of Solution C and Solution D (9:1). Initially add about 2 mL of Solution D and then add Solution D or Solution C to maintain a final composition of Solution C and Solution D in the ratio of 9:1. Sonicate briefly for complete dissolution.
Sample solution: 0.6 mg/mL of Doxazosin Mesylate in a mixture of Solution C and Solution D (9:1). Initially add about 2 mL of Solution D and then add Solution D or Solution C to maintain a final composition of Solution C and Solution D in the ratio of 9:1. Sonicate briefly for complete dissolution,
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4-mm × 25-cm; 5-µm packing L7
Column temperature: 35°
Flow rate: 0.8 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 2 between doxazosin related compound A and doxazosin related compound B, System suitability solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of doxazosin mesylate (C23H25N5O5 · CH4O3S) in the portion of Doxazosin Mesylate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Doxazosin Mesylate RS in the Standard solution (mg/mL)
Cu = concentration of Doxazosin Mesylate in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%
Change to read:
ORGANIC IMPURITIES
Solution A, Solution B, Solution C, Solution D, Mobile phase, System suitability solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 0.0015 mg/mL, each of USP Doxazosin Mesylate RS, USP Doxazosin Related Compound A RS, USP Doxazosin Related Compound B RS. USP Doxazosin Related Compound C RS. USP Doxazosin Related Compound D RS, USP Doxazosin Related Compound E RS, USP Doxazosin Related Compound F. RS, USP Terazosin Related Compound A RS, and USP Terazosin Related Compound C RS in a mixture of Solution C and Solution D. Initially dissolve USP Doxazosin Related Compounds A, B, C, D, E, and F RS and USP Terazosin Related Compounds A and C RS in approximately 2 mL of Solution D only and then add Solution D or Solution C to maintain a final composition of Solution C and Solution D in the ratio of 9:1. Sonicate briefly for complete dissolution.
Sample solution: 0.6 mg/mL of Doxazosin Mesylate in a mixture of Solution C and Solution D (9:1). Initially add about 2 mL of Solution D and then add Solution D or Solution C to maintain a final composition of Solution C and Solution D in the ratio of 9:1. Sonicate briefly for complete dissolution.
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 2 between doxazosin related compound A and doxazosin related compound B, System suitability solution
Relative standard deviation: NMT 10% for all peaks, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Doxazosin Mesylate taken:
Result = (ru /rs ) × (Cs /Cu ) × (Mr1 /Mr2 ) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of each impurity or doxazosin mesylate (for calculating unspecied impurities) from the Standard solution
Cs = concentration of the corresponding USP Reference Standard (ERR 1-Nov-2022) or USP Doxazosin Mesylate RS (for calculating unspecied impurities) in the Standard solution (mg/mL)
Cu = concentration of Doxazosin Mesylate in the Sample solution (mg/mL)
Mr1 = molecular weight of the corresponding impurity in the sample (see Table 2)
Mr2 = molecular weight of corresponding impurity Reference Standard (see Table 2)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Molecular Weight of Reference Standard (Mr1) | Molecular Weight of Corresponding Impurity in Sample (Mr2) | Acceptance Criteria, NMT (%) |
| Terazosin related compound Aa,b | 0.20 | 362.25 | 481.55 | 0.3 |
| Doxazosin related compound Ac,b | 0.44 | 248.28 | 344.39 | 0.25 |
| Doxazosin related compound Bd | 0.48 | 222.20 | 222.20 | 0.25 |
| Doxazosin related compound Ce,b | 0.56 | 239.66 | 335.77 | 0.25 |
| Terazosin related compound Cf,b | 0.61 | 565.45 | 684.75 | 0.25 |
| Doxazosin related compound Dg | 0.83 | 180.16 | 180.16 | 0.25 |
| Doxazosin mesylate | 1.00 | - | - | - |
| Doxazosin related compound Eh | 1.45 | 259.09 | 259.09 | 0.25 |
| Doxazosin related compound Fi | 1.55 | 410.42 | 410.42 | 0.25 |
| Any unspecied impurity | - | - | - | 0.10 |
| Total impurities | - | - | - | 1.0 |
a 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)piperazine, dihydrochloride.
b This impurity exists as a mesylate salt in the sample.
c N-1,4-Benzodioxane-2-carbonyl Piperazine.
d 6,7-Dimethoxyquinazoline-2,4-dione.
e 2-Chloro-4-amino-6,7-dimethoxy quinazoline.
f 1,4-Bis(4-amino-6,7-dimethoxy-2-quinazolinyl)piperazine, dihydrochloride.
g 1,4-Benzodioxane-2-carboxylic acid.
h 2,4-Dichloro-6,7-dimethoxyquinazoline.
i N,N′-Bis(1,4-benzodioxane-2-carbonyl)piperazine.
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Sample: 1.0 g
Analysis: Dry the Sample under vacuum at 105° for 4 h.
Acceptance criteria: NMT 2.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers, and store below 30°.
USP Reference Standards 〈11〉
USP Doxazosin Mesylate RS
USP Doxazosin Related Compound A RS
N-1,4-Benzodioxane-2-carbonyl piperazine.
C13H16N2O3 248.28
USP Doxazosin Related Compound B RS
6,7-Dimethoxyquinazoline-2,4-dione.
C10H10N2O4 222.20
USP Doxazosin Related Compound C RS
2-Chloro-4-amino-6,7-dimethoxyquinazoline.
C10H10ClN3O2 239.66
USP Doxazosin Related Compound D RS
1,4-Benzodioxane-2-carboxylic acid.
C9H8O4 180.16
USP Doxazosin Related Compound E RS
2,4-Dichloro-6,7-dimethoxyquinazoline.
C10H8Cl2N2O2 259.09
USP Doxazosin Related Compound F RS
N,N′-Bis(1,4-benzodioxane-2-carbonyl)piperazine.
C22H22N2O6 410.42
USP Terazosin Related Compound A RS
1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)piperazine, dihydrochloride.
C14H19N5O2 · 2HCl 362.25
USP Terazosin Related Compound C RS
1,4-Bis(4-amino-6,7-dimethoxy-2-quinazolinyl)piperazine, dihydrochloride.
C24H28N8O4 · 2HCl 565.45


