Dolasetron Mesylate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
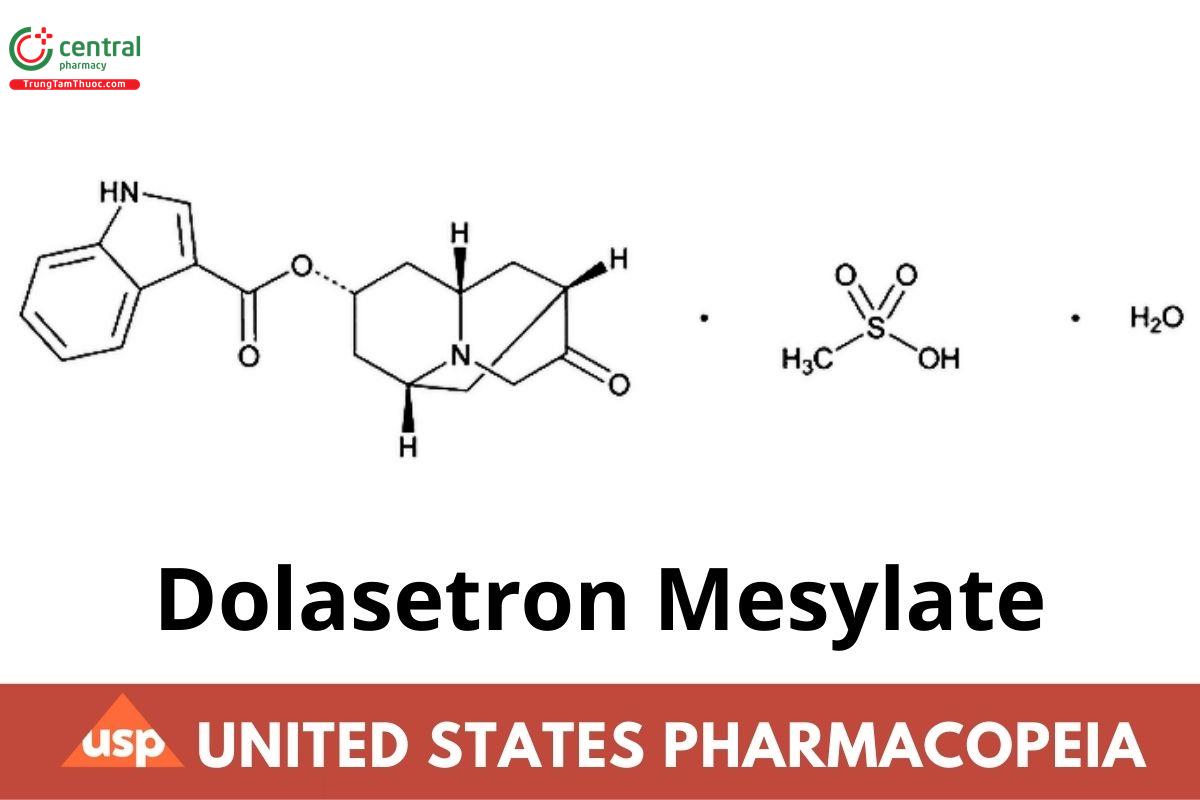
C19H20N2O3 · CH4O2S · H2O 438.49
1H-Indole-3-carboxylic acid, octahydro-3-oxo-2,6-methano-2H -quinolizin-8-yl ester, (2α,6α,8α,9aβ)-, monomethanesulfonate monohydrate;
Indole-3-carboxylic acid, ester with (8r)-hexahydro-8-hydroxy-2,6-methano-2H-quinolizin-3(4H)-one, monomethanesulfonate monohydrate CAS RN: 878143-33-0; UNII: U3C8E5BWKR.
Anhydrous CAS RN: 115956-13-3; UNII: 71YF100S3H.
1 DEFINITION
Dolasetron Mesylate contains NLT 98.0% and NMT 102.0% of C19H20N2O3 · CH4O2S · H2O, calculated on the as-is basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. PROCEDURE
Sample solution: 1 mg/mL
Analysis: Transfer 5-10 mg of 5,5'-methylenedisalicylic acid to a clean crucible, and heat in an oven at 150° for 5 min. Remove from the oven, and add 10 drops of the Sample solution. Return to the oven, and evaporate to dryness.
Acceptance criteria: A red or pink color (presence of methanesulfonic acid) develops in the white residue.
3 ASSAY
Change to read:
PROCEDURE
Solution A: Add 0.19 mL. of triethylamine to each 450 mL of acetonitrile. (ERR 1-Jun-2020)
Mobile phase: Solution A. (ERR 1-Jun-2020) water, and 1 M ammonium formate (450:440:110) (ERR 1-Jun-2020)
Standard solution: 0.04 mg/mL and 0.004 mg/mL respectively of USP Dolasetron Mesylate RS and indole-3-carboxylic acid in Mobile phase
Sample solution: 0.04 mg/mL of Dolasetron Mesylate in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 285 nm
Column: 4.6-mm x 15-cm; packing L1
Flow rate: 1 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 4 between indole-3-carboxylic acid and dolasetron mesylate
Tailing factor: NMT 1.8
Relative standard deviation: NMT 1.5% for replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C19H20N2O3 · CH4O2S · H2O in the Dolasetron Mesylate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Dolasetron Mesylate RS in the Standard solution (mg/mL)
Cu = concentration of Dolasetron Mesylate in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the as-is basis
4 IMPURITIES
Organic Impurities
Procedure
0.01 M Dibasic ammonium phosphate solution: 1.32 g/L of dibasic ammonium phosphate. Adjust with 2.0 M phosphoric acid to a pH of7.0.
Diluent: Acetonitrile and water (1:4)
Solution A: Acetonitrile and 0.01 M Dibasic ammonium phosphate solution (53:1000), ltered and degassed
Solution B: Acetonitrile and 0.01 M Dibasic ammonium phosphate solution (795:295), ltered and degassed
Mobile phase: See the gradient table below.
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 100 | 0 |
| 28 | 0 | 100 |
| 38 | 0 | 100 |
| 40 | 100 | 0 |
| 50 | 100 | 0 |
System suitability solution: 0.004 mg/mL and 0.03 mg/ml, respectively, of indole and USP Dolasetron Mesylate RS in Diluent
Standard solution A: 0.03 mg/mL of USP Dolasetron Mesylate RS in Diluent
Polasetron Standard solution B: 6 mg/mL and 0.0072 mg/mL, respectively, of USP Dolasetron Mesylate RS and USP Dolasetron Mesylate Related
Compound A RS in Diluent
Sample solution: 6 mg/mL of Dolasetron Mesylate in Diluent
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4.6-mm x 25-cm; packing L7
Flow rate: 1.5 mL/min
Injection size: 100 µL
System suitability
Suitability requirements
Resolution: NLT 1.5 between the first eluting peak, indole, and the second eluting peak, dolasetron mesylate from the System suitability solution. [NOTE-If the dolasetron mesylate peak is found to elute before the indole peak, condition the column as follows: fill up the column with Solution A, plug the column, and place the column in a convection oven at 105° for about 16 h. Retest the column.]
Relative standard deviation: NMT 5.0% for replicate injections of Standard solution A
Analysis
Samples: Standard solution A, Standard solutionB, and Sample solution
Calculate the percentage of dolasetron mesylate related compound A in the Dolasetron Mesylate taken:
Result = (ru /rs ) × (Cs /Cu ) × (Mr1 /Mr2 ) × 100
ru = peak response of dolasetron mesylate related compound A from the Sample solution
rs = peak response of dolasetron mesylate related compound A from the Standard solution B
Cs = concentration of USP Dolasetron Mesylate Related Compound A RS in the Standard solution B (mg/mL)
Cu = concentration of Dolasetron Mesylate in the Sample solution (mg/mL)
Mr1 = molecular weight of dolasetron mesylate related compound A base, 181.2
Mr2 = molecular weight of dolasetron mesylate related compound A hydrochloride, 217.8
Calculate the percentage of each impurity (other than dolasetron mesylate related compound A) in the portion of Dolasetron Mesylate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of dolasetron mesylate from the Standard solution A
Cs = concentration of USP Dolasetron Mesylate RS in the Standard solution A (mg/mL)
Cu = concentration of Dolasetron Mesylate in the Sample solution (mg/mL)
Acceptance criteria
Individual impurities: NMT 0.1%
Total impurities: NMT 0.3%
[Note—The reporting level for impurities is 0.05%.]
5 SPECIFIC TESTS
WATER DETERMINATION, Method la(921): Between 3.5% and 4.7%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers, protected from light.
USP REFERENCE STANDARDS (11)
USP Dolasetron Mesylate RS
USP Dolasetron Mesylate Related Compound A RS
Hexahydro-8-hydroxy-2,6-methano-2H-quinolizin-3 (4H)-one, hydrochloride.


