Docetaxel
If you find any inaccurate information, please let us know by providing your feedback here
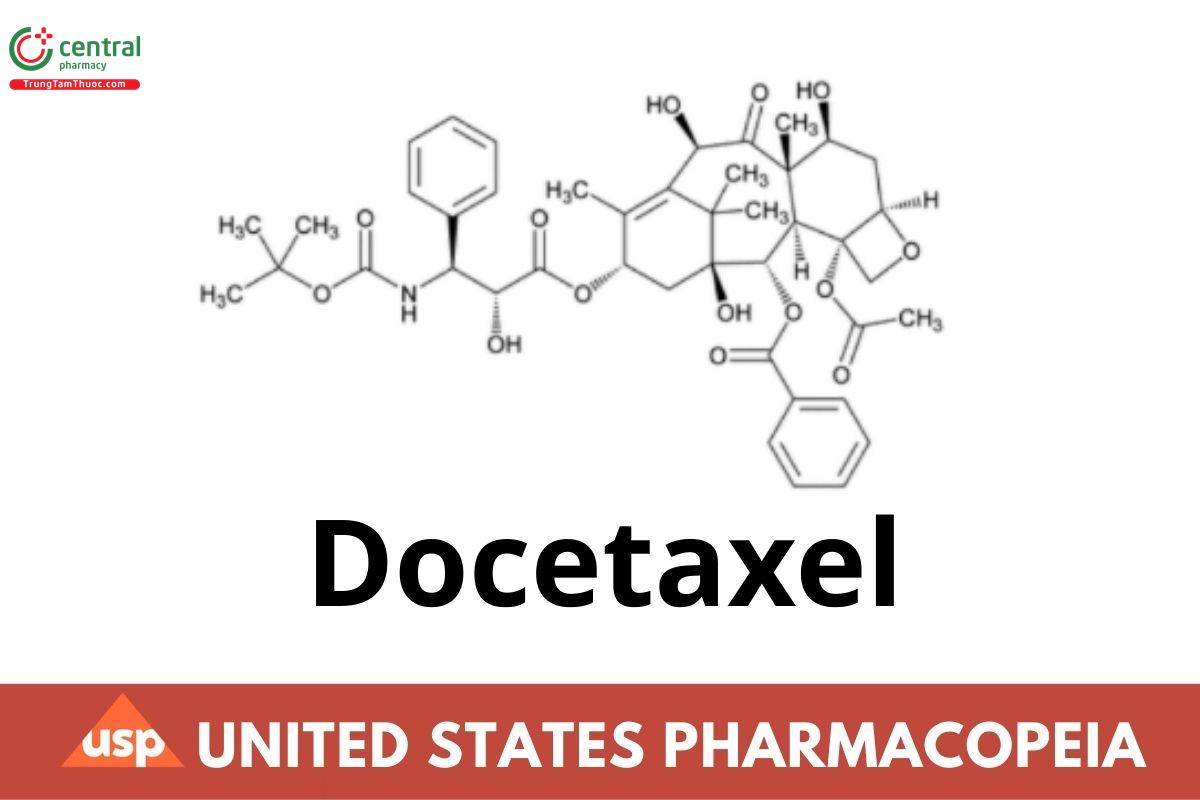
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Docetaxel contains NLT 97.5% and NMT 102.0% of docetaxel (C43H53NO14), calculated on the anhydrous and solvent-free basis. [Caution—Docetaxel is cytotoxic. Great care should be taken to prevent inhaling particles of Docetaxel and exposing the skin to it.]
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A, 197K, 197M, or 197S
[Note—Use a solution containing 60 mg/mL of Docetaxel in methylene chloride for 〈197S〉.]
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Solution A: Water
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 72 | 28 |
| 9.0 | 72 | 28 |
| 39.0 | 28 | 72 |
| 39.1 | 72 | 28 |
| 50 | 72 | 28 |
Diluent: Acetonitrile, water, and glacial acetic acid (100:100:0.1)
System suitability solution: 1 mg/mL of USP Docetaxel Identification RS in Diluent. [Note—USP Docetaxel Identification RS contains docetaxel and small amounts of 2-debenzoxyl 2-pentenoyl docetaxel, 6-oxodocetaxel, 4-epidocetaxel, and 4-epi-6-oxodocetaxel. See Table 2.]
Standard solution: 1.0 mg/mL of USP Docetaxel RS prepared as follows. Transfer a quantity of USP Docetaxel RS to a suitable volumetric flask, dissolve in alcohol equivalent to about 5% of the final volume, and dilute with Diluent to volume.
Sample solution: 1.0 mg/mL of Docetaxel prepared as follows. Transfer a quantity of Docetaxel to a suitable volumetric flask, dissolve in alcohol equivalent to about 5% of the final volume, and dilute with Diluent to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 232 nm
Column: 4.6-mm × 15-cm; 3.5-μm packing L1
Temperatures
Autosampler: 10°
Column: 45°
Flow rate: 1.2 mL/min
Injection volume: 10 μL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 3.5 between 2-debenzoxyl 2-pentenoyl docetaxel and docetaxel, System suitability solution
Relative standard deviation: NMT 1.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of docetaxel (C43H53NO14) in the portion of Docetaxel taken:
Result = (rU/rS) × (CS/CU) × 100
rU= peak response from the Sample solution
rS= peak response from the Standard solution
CS= concentration of USP Docetaxel RS in the Standard solution (mg/mL)
CU= concentration of Docetaxel in the Sample solution (mg/mL)
Acceptance criteria: 97.5%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
Organic Impurities, Procedure 1
[Note—On the basis of the synthetic route, perform either Procedure 1 or Procedure 2. Procedure 1 is recommended if 10-deacetyl baccatin and 2-debenzoxyl 2-pentenoyl docetaxel are specified impurities. Procedure 2 is recommended if O-BOC N-pyruvyl docetaxel is a specified impurity.]
Mobile phase, Diluent,(ERR 1-Jun-2024) System suitability solution, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Sensitivity solution: 0.5 μg/mL of USP Docetaxel RS in Diluent from the Standard solution
System suitability
Samples: System suitability solution and Sensitivity solution
Suitability requirements
Resolution: NLT 3.5 between 2-debenzoxyl 2-pentenoyl docetaxel and docetaxel, System suitability solution
Signal-to-noise ratio: NLT 10 for the docetaxel peak, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Docetaxel taken:
Result = (rU/rT) × (1/F) × 100
rU= peak response of each individual impurity from the Sample solution
rT= sum of all peak responses from the Sample solution
F = relative response factor for each individual impurity (see Table 2)
Acceptance criteria: See Table 2. Disregard any peaks less than 0.05%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| 10-Deacetyl baccatin (if present)a,b | 0.25 | 1.5 | 0.15 |
N-Formyl impurity (if present)b,c | 0.57 | 1.1 | 0.15 |
2-Debenzoxyl 2-pentenoyl docetaxel | 0.97 | 0.63 | 0.5 |
| Docetaxel | 1.00 | - | - |
| 6-Oxodocetaxel | 1.08 | 1.0 | 0.3 |
| 4-Epidocetaxel | 1.13 | 1.0 | 0.3 |
| 4-Epi-6-oxodocetaxel | 1.18 | 1.0 | 0.2 |
6-Dichloroethoxycarbonyl docetaxel (if present)b,d | 1.31 | 0.82 | 0.15 |
| Any unspecified impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 1.0 |
a (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one 12b-acetate, 12-benzoate.
b If possible from the manufacturing process.
c (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-Acetoxy-9-(((2R,3S)-3-formamido-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate.
d (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6-{[(2,2-Dichloroethoxy)carbonyl]oxy}-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,9,11,12,12b-pentahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one 12b-acetate, 12-benzoate, 9-ester with (2R,3S)-N-tert-butoxycarbonyl-3-phenylisoserine.
Organic Impurities, Procedure 2
Solution A: Water
Solution B: Acetonitrile
Mobile phase: See Table 3.
Table 3
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 65 | 35 |
| 25 | 45 | 55 |
| 35 | 20 | 80 |
| 45 | 20 | 80 |
| 45.1 | 65 | 35 |
| 53 | 65 | 35 |
System suitability solution: 1 mg/mL of USP Docetaxel System Suitability Mixture RS in acetonitrile. [Note—USP Docetaxel System Suitability
Mixture RS contains docetaxel and a small amount of 6-oxodocetaxel, O-BOC N-pyruvyl docetaxel, 4-epidocetaxel, and 4-epi-6-oxodocetaxel. See Table 4.]
Standard solution: 5.0 μg/mL of USP Docetaxel RS in acetonitrile
Sample solution: 1.0 mg/mL of Docetaxel in acetonitrile
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 15-cm; 3.5-μm packing L1
Temperatures
Column: 40°
Sample: 4°
Flow rate: 1.2 mL/min
Injection volume: 10 μL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 2.0 between O-BOC N-pyruvyl docetaxel and 4-epidocetaxel
Tailing factor: 0.8–1.5 for the docetaxel peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Docetaxel taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU= peak area of each individual impurity from the Sample solution
rS= peak area of docetaxel from the Standard solution
CS= concentration of USP Docetaxel RS in the Standard solution (mg/mL)
CU= concentration of Docetaxel in the Sample solution (mg/mL)
F = relative response factor for each individual impurity (see Table 4)
Acceptance criteria: See Table 4. Disregard any peaks less than 0.05%.
Table 4
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Docetaxel | 1.0 | - | - |
| 6-Oxodocetaxel | 1.19 | 1.1 | 0.15 |
| O-BOC N-pyruvyl docetaxela | 1.24 | 0.8 | 0.15 |
| 4-Epidocetaxel | 1.29 | 0.96 | 0.15 |
| 4-Epi-6-oxodocetaxel | 1.42 | 1.2 | 0.15 |
| Any unspecified impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 2.0 |
a (2aR,4R,4aS,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4, 9,11,12,12b-pentahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5,6-dione 12b-acetate, 12-benzoate, 9-ester with (2R,3S)-2-(tert-butoxycarbonyloxy)-3-(2-oxopropanamido)-3-phenylpropanoic acid.
5 SPECIFIC TESTS
Microbial Enumeration Tests 〈61〉andTests for Specified Microorganisms 〈62〉: The total aerobic microbial limit does not exceed 102cfu/g. The total molds and yeasts count does not exceed 10 cfu/g.
Bacterial Endotoxins Test 〈85〉: It contains NMT 0.4 USP Endotoxin Units/mg.
Water Determination 〈921〉, Method I, Method Ic: 5.0%–7.0%. If labeled as an anhydrous form: NMT 1.5%.
Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 10 mg/mL in methanol
Acceptance criteria: −39° to −41° (t = 20°), calculated on the anhydrous and solvent-free basis. If labeled as an anhydrous form: −35° to −45° (t = 20°), calculated on the as-is basis.
6 ADDITIONAL REQUIREMENTS
Labeling: Where it is an anhydrous form, the label so indicates. If a test for Organic Impurities other than Procedure 1 is used, the labeling states the test with which the article complies.
Packaging and Storage: Preserve in well-closed, light-resistant containers, and store at room temperature or between 2° and 8°.
USP Reference Standards 〈11〉
USP Docetaxel RS
USP Docetaxel Identification RS
It contains docetaxel and small amounts of the following:
2-Debenzoxyl 2-pentenoyl docetaxel: (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one 12b-acetate, 12-[(E)-2-methylbut-2-enoate], 9-ester with (2R,3S)-N-tert-butoxycarbonyl-3-phenylisoserine.
C41H55NO14 785.87
6-Oxodocetaxel: (2aR,4S,4aS,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4, 9,11,12,12b-pentahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2b]oxet-5,6-dione 12b-acetate, 12-benzoate, 9-ester with (2R,3S)-N-tert-butoxycarbonyl-3-phenylisoserine.
C43H51NO14 805.86
4-Epidocetaxel: (2aR,4R,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one 12b-acetate, 12-benzoate, 9-ester with (2R,3S)-N-tert-butoxycarbonyl-3-phenylisoserine.
C43H53NO14 807.88
4-Epi-6-oxodocetaxel: (2aR,4R,4aS,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4, 9,11,12,12b-pentahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5,6-dione 12b-acetate, 12-benzoate, 9-ester with (2R,3S)-N-tert-butoxycarbonyl-3-phenylisoserine.
C43H51NO14 805.86
USP Docetaxel System Suitability Mixture RS
Contains docetaxel and a small amount of 6-oxodocetaxel, O-BOC N-pyruvyl docetaxel, 4-epidocetaxel, and 4-epi-6-oxodocetaxel.


