Dipyridamole
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
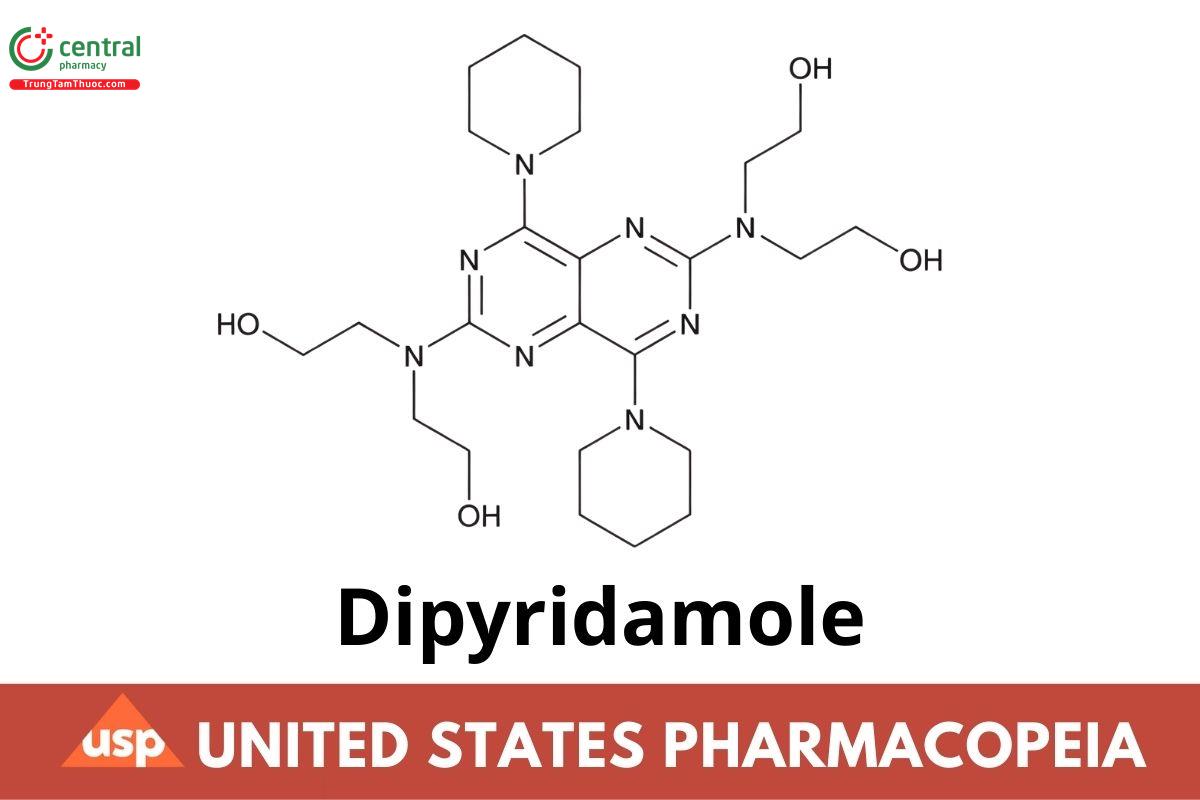
C24H40N8O4 504.63
Ethanol, 2,2',2",2"'-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl) dinitrilo]tetrakis-;
2,2',2",2"'-[(4,8-Dipiperidinopyrimido [5,4-d]pyrimidine-2,6-diyl) dinitrilo tetraethanol CAS RN®: 58-32-2; UNII: 64ALC7F90C.
1 DEFINITION
Dipyridamole contains NLT 98.0% and NMT 102.0% of dipyridamole (C24H40N8O4), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K ▲(CN1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Buffer: 0.77 g/L of ammonium acetate. Adjust with glacial acetic acid to a pH of 4.0.
Mobile phase: Methanol and Buffer (700:300)
Standard solution: 0.2 mg/mL of USP Dipyridamole RS in methanol
Sample solution: 0.2 mg/mL of Dipyridamole in methanol
3.1.1 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 288 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Flow rate: 1.2 mL/min
Injection volume: 10 µL
Run time: NLT 1.7 times the retention time of dipyridamole
3.1.2 System suitability
Sample: Standard solution
3.1.3 Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 0.73%
3.1.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of dipyridamole (C24H40N8O4) in the portion of Dipyridamole taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of dipyridamole from the Sample solution
rS = peak response of dipyridamole from the Standard solution
CS = concentration of USP Dipyridamole RS in the Standard solution (mg/mL)
CU = concentration of Dipyridamole in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281): NMT 0.1%
4.2 ORGANIC IMPURITIES
Solution A: 10 mM ammonium formate. Adjust with formic acid to a pH of 5.0.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0.0 | 80 | 20 |
| 2.0 | 80 | 20 |
| 17.0 | 5 | 95 |
| 19.0 | 5 | 95 |
| 21.0 | 80 | 20 |
| 25.0 | 80 | 20 |
Standard solution: 0.5 µg/mL each of USP Dipyridamole RS. USP Dipyridamole Related Compound A RS, USP Dipyridamole Related Compound B RS, USP Dipyridamole Related Compound C RS, USP Dipyridamole Related Compound D RS. USP Dipyridamole Related Compound E RS, and USP Dipyridamole Related Compound F RS in methanol
Sample solution: 500 µg/mL of Dipyridamole in methanol
4.2.1 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 295 nm
Column: 2.1-mm × 15-cm; 1.7-µm packing L1
Column temperature: 45°
Flow rate: 0.3 mL/min
Injection volume: 2 µL
4.2.2 System suitability
Sample: Standard solution
4.2.3 Suitability requirements
Resolution: NLT 4.0 between dipyridamole related compound D and dipyridamole
Relative standard deviation: NMT 2.8%
4.2.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each specified impurity in the portion of Dipyridamole taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of each specified impurity from the Sample solution
rS = peak response of corresponding USP Reference Standard from the Standard solution
CS = concentration of corresponding USP Reference Standard in the Standard solution (µg/mL)
CU = concentration of Dipyridamole in the Sample solution (µg/mL)
Calculate the percentage of each unspecified impurity in the portion of Dipyridamole taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of each unspecified impurity from the Sample solution
rS = peak response of dipyridamole from the Standard solution S
CS = concentration of USP Dipyridamole RS in the Standard solution (µg/mL)
CU = concentration of Dipyridamole in the Sample solution (µg/mL)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Dipyridamole related compound B | 0.6 | 0.50 |
| Dipyridamole related compound F | 0.7 | 0.1 |
| Dipyridamole related compound D | 0.9 | 0.2 |
| Dipyridamole | — | — |
| Dipyridamole related compound E | 1.2 | 0.2 |
| Dipyridamole related compound C | 1.5 | 0.1 |
| Dipyridamole related compound A | 1.9 | 0.50 |
| Any unspecified impurity | — | 0.10 |
| Total impurities | — | 1.0 |
5 SPECIFIC TESTS
5.1 CHLORIDE
Sample: 500 mg
Acceptance criteria: No turbidity or precipitate is produced.
5.2 LOSS ON DRYING (731)
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers. Store at room temperature.
USP REFERENCE STANDARDS (11).
USP Dipyridamole RS
USP Dipyridamole Related Compound A RS
2,2'-([4,6,8-Tri(piperidin-1-yl)pyrimido[5,4-d]pyrimidin-2-yl]azanediyl) diethanol.
C25H40N8O2 484.64
USP Dipyridamole Related Compound B RS
2,2',2'',2''',2''',2'''''-([8-(Piperidin-1-yl)pyrimido [5,4-d]pyrimidine-2,4,6-triyl]tris(azanetriyl))hexaethanol.
C23H40N8O6 524.61
USP Dipyridamole Related Compound C RS
2,2'-([6-Chloro-4,8-di(piperidin-1-yl)pyrimido [5,4-d]pyrimidin-2-yl]azanediyl)diethanol.
C20H30CIN7O2 435.95
USP Dipyridamole Related Compound D RS
2,2'-((6-[(2-Hydroxyethyl)amino]-4,8-di(piperidin-1-yl)pyrimido [5,4-d]pyrimidin-2-yl)azanediyl) diethanol.
C22H36N8O3 460.57
USP Dipyridamole Related Compound E.RS
2,2',2",2"'-([6,8-Di(piperidin-1-yl)pyrimido [5,4-d]pyrimidine-2,4-diyl]bis(azanetriyl)}tetraethanol.
C22H40N8O4 504.63
USP Dipyridamole Related Compound F. RS
2,2',2",2"'-({4-[(2-Hydroxyethyl)amino]-8-(piperidin-1-yl)pyrimido [5,4-d]pyrimidine-2,6-diyl)bis(azanetriyl))tetraethanol.
C21H36N8O5 480.56


