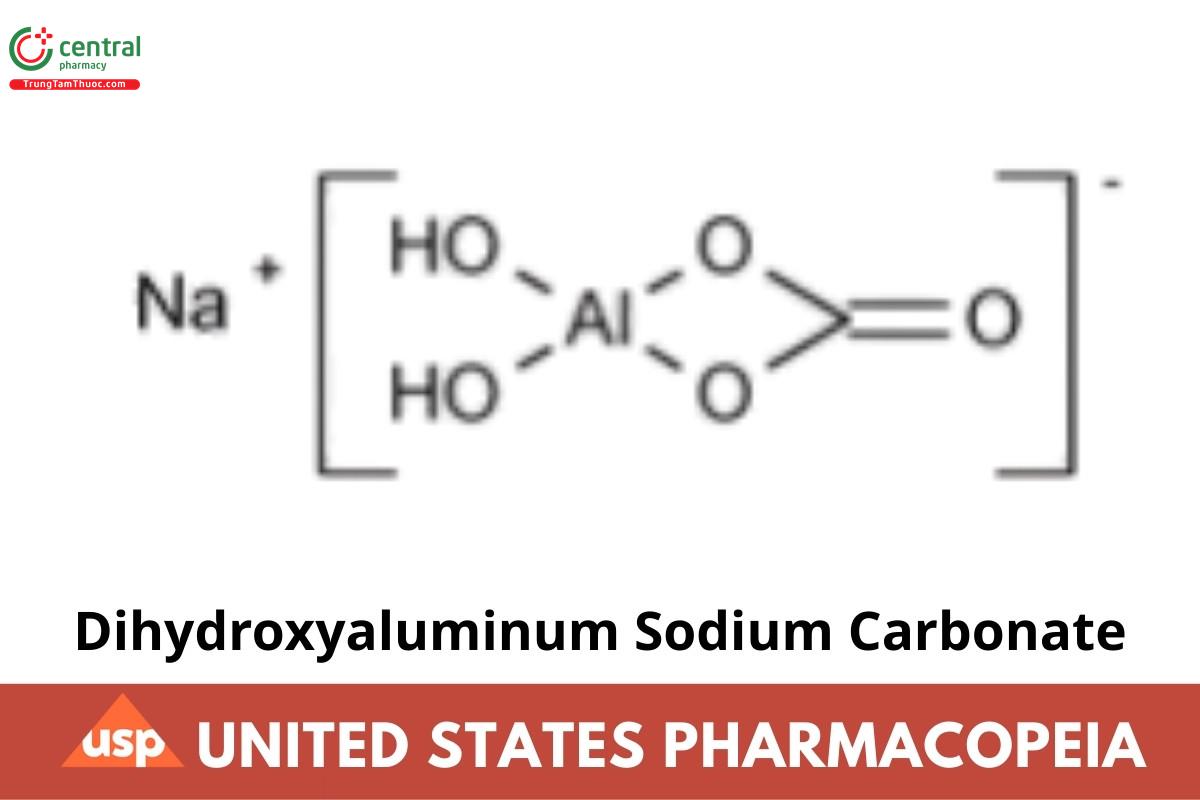
Dihydroxyaluminum Sodium Carbonate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Dihydroxyaluminum Sodium Carbonate contains NLT 98.3% and NMT 107.9% of dihydroxyaluminum sodium carbonate [NaAl(OH)2CO3], calculated on the dried basis.
2 IDENTIFICATION
2.1 A.
Sample solution: Combine 1 g with 20 mL of 3 N hydrochloric acid.
Acceptance criteria: The sample dissolves with effervescence.
2.2 B.
Identification Tests—General, Aluminum 〈191〉
Sample: Sample solution prepared in Identication test A
Acceptance criteria: Meets the requirements
2.3 C.
The Sample solution, prepared and tested as directed in the test for Sodium Content, exhibits a signicant absorption at the sodium emission line at 589.0 nm.
3 ASSAY
3.1 Procedure
Edetate disodium titrant: Dissolve 18.6 g of edetate disodium in water to make 500 mL, and standardize as directed in Reagents, Volumetric Solutions, Edetate Disodium, Twentieth-Molar (0.05 M).
Sample: 300 mg undried
Analysis: Transfer the Sample to a 250-mL beaker, add 10 mL of 2 N sulfuric acid, cover the beaker, heat to 80° for 5 min, and boil for 1 min. Add 30.0 mL of 0.1 M edetate disodium VS, again boil for 1 min, cool, and then add 10 mL of acetic acid–ammonium acetate buffer TS, 50 mL of acetone, and 2 mL of dithizone TS. Using a pH meter, adjust with the addition of ammonium hydroxide or dilute sulfuric acid to a pH of 4.5. Titrate with 0.05 M zinc sulfate VS, maintaining the pH of 4.5 by the addition of ammonium hydroxide as necessary, to an orange pink color. Perform a blank determination, and make any necessary correction. Each mL of 0.1 M Edetate disodium titrant is equivalent to 14.40 mg of dihydroxyaluminum sodium carbonate [NaAl(OH)2CO3].
Acceptance criteria: 98.3%–107.9% on the dried basis
4 IMPURITIES
Change to read:
4.1 Mercury 〈261〉, Procedures, Procedure 2 (CN 1-Jun-2023)
Sample solution: 2.0 g in 35 mL of 1 N sulfuric acid
Acceptance criteria: NMT 1 ppm
4.2 Isopropyl Alcohol
Isopropyl alcohol-free dihydroxyaluminum sodium carbonate: Use a portion of Dihydroxyaluminum Sodium Carbonate that has been previously tested as directed in this section and found to be free of isopropyl alcohol.
Sodium chloride solution: 0.2 g/mL in water
Standard stock solution: 20 mg/mL of isopropyl alcohol in Sodium chloride solution
Standard solution A: 0.4 mg/mL of isopropyl alcohol in Sodium chloride solution from Standard stock solution Standard solution B: 0.8 mg/mL of isopropyl alcohol in Sodium chloride solution from Standard stock solution Standard solution C: 1.0 mg/mL of isopropyl alcohol in Sodium chloride solution from Standard stock solution Standard solution D: 1.2 mg/mL of isopropyl alcohol in Sodium chloride solution from Standard stock solution Headspace containers: Use suitable 20-mL containers capable of being tightly closed with an inert septum and a metallic crimp cap.
Standard preparations: To four separate 20-mL Headspace containers, add 1.0 g of Isopropyl alcohol-free dihydroxyaluminum sodium carbonate. To the containers add, respectively, 10.0 mL of the appropriate Standard solution. These containers contain about 4, 8, 10, and 12 mg of isopropyl alcohol, respectively. [Note—Keep the containers cool until sealed.] Seal the containers, place in a water bath maintained at 70°, and allow to stand for 1 h.
Sample preparation: Transfer 1.0 g of the Dihydroxyaluminum Sodium Carbonate to a Headspace container, and add 10.0 mL of Sodium chloride solution. [Note—Keep the container cool until sealed.] Seal the container, place in a water bath maintained at 70°, and allow to stand for 1 h.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.9-m × 3-mm; packed with support S3
Temperatures
Column: 180°
Injection port: 200°
Detector: 250°
Injection volume: 1 mL of gaseous phase
System suitability
Samples: Standard preparations containing 10 mg per container
Suitability requirements
Relative standard deviation: NMT 4% for replicate injections
Analysis
Samples: Standard preparations and Sample preparation
[Note—Use peak areas where peak responses are indicated.]
Using a gas-tight syringe, separately inject equal volumes of the gaseous headspace of the Standard preparations and the Sample preparation into the gas chromatograph. Record the chromatograms, and measure the peak responses. Determine, based on a retention time comparison, if isopropyl alcohol is detected in the Sample preparation. Plot the responses of the Standard preparations versus the content, in mg, of isopropyl alcohol in each container, draw the straight line best tting the plotted points, and calculate the correlation coecient for the line. A suitable system is one that yields a line having a correlation coecient of NLT 0.99. From the graph, determine the total amount, TU, in mg, of isopropyl alcohol in the Sample preparation.
Calculate the percentage of isopropyl alcohol in the Dihydroxyaluminum Sodium Carbonate taken:
Result = 0.1 × (TU/WU)
TU = total amount of isopropyl alcohol in the Sample preparation (mg)
WU = weight of the Dihydroxyaluminum Sodium Carbonate taken (g)
Acceptance criteria: NMT 1.0%
5 SPECIFIC TESTS
5.1 Sodium Content
Potassium chloride solution: 38 mg/mL of potassium chloride in water
Sodium chloride stock solution: 25.42 µg/mL of sodium chloride in water (10.0 µg/mL of sodium) from sodium chloride previously dried at 105° for 2 h
Standard solution A: 0.5 µg/mL of sodium from Sodium chloride stock solution prepared as follows. On the day of use, transfer 4.0 mL of 1 N hydrochloric acid and 10.0 mL of Potassium chloride solution to a 100-mL volumetric ask. Add 5.0 mL of Sodium chloride stock solution and dilute with water to volume.
Standard solution B: 1.0 µg/mL of sodium from Sodium chloride stock solution prepared as follows. On the day of use, transfer 4.0 mL of 1 N hydrochloric acid and 10.0 mL of Potassium chloride solution to a 100-mL volumetric ask. Add 10.0 mL Sodium chloride stock solution and dilute with water to volume.
Sample solution: Transfer 250 mg of Dihydroxyaluminum Sodium Carbonate, previously dried, to a 200-mL volumetric ask. Add 40 mL of 1 N hydrochloric acid, and boil for 1 min. Cool, and dilute with water to volume. Transfer 10.0 mL of this solution to a 100-mL volumetric ask, and dilute with water to volume. Transfer 5.0 mL of this solution to a 100-mL volumetric ask containing 4.0 mL of 1 N hydrochloric acid and 10.0 mL of Potassium chloride solution, and dilute with water to volume.
Blank solution: Pipet 4 mL of 1 N hydrochloric acid and 10.0 mL of Potassium chloride solution into a 100-mL volumetric ask, and dilute with water to volume.
Instrumental conditions
(See Atomic Absorption Spectroscopy 〈852〉.)
Analytical wavelength: Sodium emission line at 589.0 nm
Lamp: Sodium hollow-cathode
Flame: Air–acetylene
Blank: Blank solution
Analysis
Samples: Standard solution A, Standard solution B, Sample solution, and Blank solution
Plot the absorbances of the Standard solutions versus the concentrations, in µg/mL of sodium, and draw a straight line between the plotted points. From the graph so obtained, determine the concentration, C, in µg/mL of sodium in the Sample solution. Calculate the percentage of sodium in the portion of Dihydroxyaluminum Sodium Carbonate taken:
Result = 4000 × (C/W)
C = concentration of sodium in the Sample solution (µg/mL)
W = weight of Dihydroxyaluminum Sodium Carbonate taken (mg)
Acceptance criteria: 15.2%–16.8%
5.2 Acid-Neutralizing Capacity 〈301〉
Sample: 425 mg of undried material
Analysis: Proceed as directed using the Sample. Each mg of dihydroxyaluminum sodium carbonate [NaAl(OH) CO ] has an expected acid 2 3
neutralizing capacity of 0.0278 mEq.
Acceptance criteria: NLT 75.0% of the expected mEq value, calculated in relation to the results of the Assay
5.3 pH 〈791〉: 9.9–10.2 in a suspension (1 in 25)
5.4 Loss on Drying 〈731〉
Sample: Dry at 130° to constant weight.
Acceptance criteria: NMT 14.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.


