Dihydroxyaluminum Aminoacetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
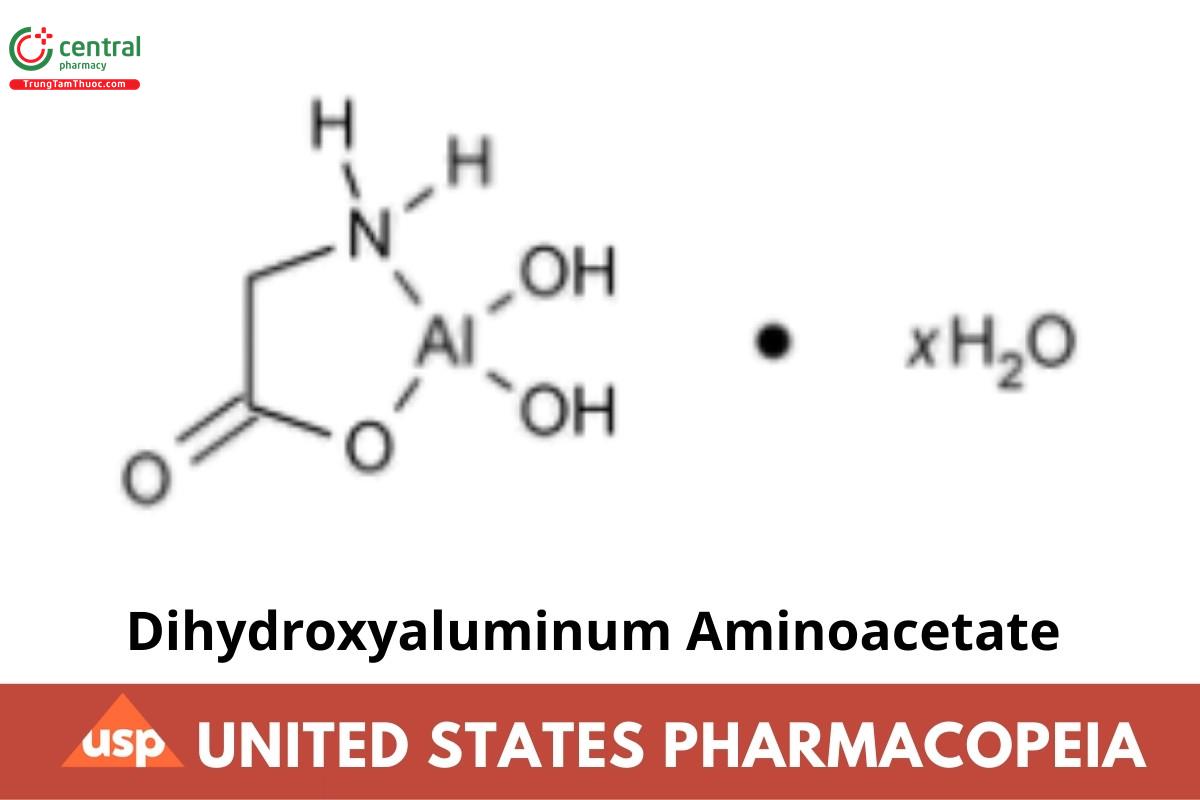
C2H6AlNO4 . H2O
Aluminum, (glycinato-N,O)dihydroxy-, hydrate.
(Glycinato)dihydroxyaluminum hydrate CAS RN®: 41354-48-7; UNII: DO250MG0W6.
Anhydrous
135.05 CAS RN®: 13682-92-3; UNII: 1K713C615K.
Dihydroxyaluminum Aminoacetate yields not less than 94.0 percent and not more than 102.0 percent of dihydroxyaluminum aminoacetate (C2H6AlNO4), calculated on the dried basis. It may contain small amounts of aluminum oxide and of Aminoacetic Acid.
1 Packaging and storage—
Preserve in well-closed containers.
2 Identication—
Suspend 1 g in 25 mL of water, add hydrochloric acid, dropwise, until a clear solution is formed, and divide it into two equal parts for the following tests.
A: One portion of the solution responds to the tests for Aluminum 〈191〉.
B: To the other portion of the solution add 1 drop of liqueed phenol and 5 mL of sodium hypochlorite TS: a blue color is produced. pH 〈791〉: between 6.5 and 7.5, in a suspension of 1 g of it, nely powdered, in 25 mL of water.
Loss on drying 〈731〉—Dry it at 130° to constant weight: it loses not more than 14.5% of its weight.
Change to read:
Mercury 〈261〉, Procedures, Procedure 2▲ (CN 1-Jun-2023) —Transfer 2.0 g to a 100-mL beaker, and add 35 mL of 1 N sulfuric acid: the limit is 1 ppm.
3 Isopropyl alcohol—
Transfer about 5 g to a ask provided with a reux condenser, and add 100 mL of potassium permanganate solution (1 in 300) and 10 mL of sulfuric acid. Reux the mixture for 30 minutes, distill, and collect 10 mL of the distillate. To 1 mL of the distillate add 5 drops of sodium nitroferricyanide TS and 2 mL of 1 N sodium hydroxide, then add a slight excess of 6 N acetic acid: no red color is produced.
4 Nitrogen—
Determine the nitrogen content as directed under Nitrogen Determination, Method II 〈461〉, using about 100 mg, previously dried and accurately weighed. Each mL of 0.1 N sulfuric acid is equivalent to 1.401 mg of nitrogen. Not less than 9.90% and not more than 10.60% of nitrogen is found.
5 Assay—
Edetate disodium titrant—Prepare and standardize as directed in the Assay under Ammonium Alum.
Procedure—Transfer about 2.5 g of Dihydroxyaluminum Aminoacetate, accurately weighed, to a 150-mL beaker, add 15 mL of hydrochloric acid, and warm, if necessary, to dissolve the specimen completely. Transfer the solution with the aid of water to a 500-mL volumetric ask, dilute with water to volume, and mix. Transfer 20.0 mL of this solution to a 250-mL beaker, and add, with continuous stirring, 25.0 mL of Edetate disodium titrant and then 20 mL of acetic acid–ammonium acetate buffer TS. Heat the solution near the boiling point for 5 minutes, cool, and add 50 mL of alcohol and 2 mL of dithizone TS. Titrate with 0.05 M zinc sulfate VS until the color changes from green-violet to rose pink. Perform a blank determination, substituting 20 mL of water for the assay solution, and make any necessary correction. Each mL of 0.05 M Edetate disodium titrant is equivalent to 6.753 mg of C2H6AlNO4.


