Diethylcarbamazine Citrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
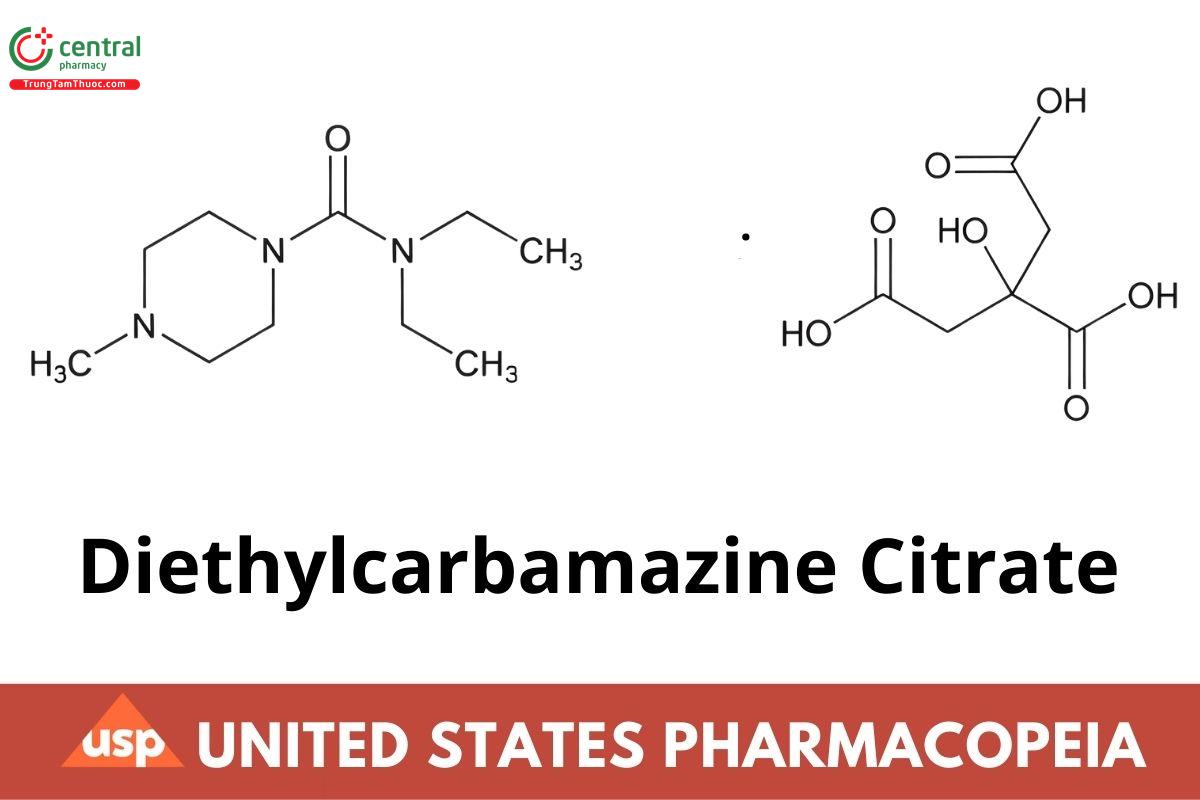
C10H21NO3 · C6H8O7 391.42
1-Piperazinecarboxamide, N,N-diethyl-4-methyl-, 2-hydroxy-1,2,3-propanetricarboxylate;
N,N-Diethyl-4-methyl-1-piperazinecarboxamide citrate (1:1) CAS RN®: 1642-54-2; UNII: OS1Z389K8S.
1 DEFINITION
Diethylcarbamazine Citrate contains NLT 98.0% and NMT 102.0% of diethylcarbamazine citrate (C10H21NO3 · C6H8O7), calculated on the anhydrous basis.
2 IDENTIFICATION
Add the following:
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay ▲(USP 1-Aug-2020)
Change to read:
B. ▲(USP 1-AUG-2020) IDENTIFICATION-ORGANIC NITROGENOUS BASES (181): Meets the requirements
Change to read:
C. ▲(USP 1-AUG-2020) IDENTIFICATION TESTS-GENERAL (191), Chemical Identification Tests, Citrate: Meets the requirements
3 ASSAY
Change to read:
3.1 PROCEDURE
Solution A: 10 mg/mL of monobasic potassium phosphate in water
Solution B: 31.24 mg/mL of monobasic potassium phosphate in water
Mobile phase: Methanol and Solution A (10:90)
Standard solution: 0.1 mg/mL of USP Diethylcarbamazine Citrate RS in Solution B
Sample solution: 0.1 mg/mL of Diethylcarbamazine Citrate in Solution B
3.1.1 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 3.9-mm x 15-cm; 5-µm packing 11
Flow rate: 0.8 mL/min
Injection volume: 20 µL
3.1.2 System suitability
Sample: Standard solution
3.1.3 Suitability requirements
Relative standard deviation: NMT 2.0% ▲▲(USP 1-Aug-2020)
3.1.4 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of diethylcarbamazine citrate (C10H21NO3 · C6H8O7) in the portion of Diethylcarbamazine Citrate taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution S
CS = concentration of USP Diethylcarbamazine Citrate RS in the Standard solution (mg/mL)
CU = concentration of Diethylcarbamazine Citrate in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%
4.1 Delete the following:
4.1.1 ORDINARY IMPURITIES (466).
Standard solution: Methanol
Sample solution: Methanol
Eluant: Methanol and ammonium hydroxide (100:1.5)
Visualization: 16 (USP 1-Aug-2020)
4.2 Add the following:
4.2.1 ORGANIC IMPURITIES, PROCEDURE 1
Impurity solution A: 0.1 mg/mL of 1-methylpiperazine in methanol
Impurity solution B: 0.1 mg/mL of 1,4-dimethylpiperazine in methanol
Standard solution: 50 mg/mL of USP Diethylcarbamazine Citrate RS in methanol
Sample solution: 50 mg/mL of Diethylcarbamazine Citrate in methanol
4.2.1.1 Chromatographic system
(See Chromatography (621), General Procedures. Thin-Layer Chromatograpby.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL
Developing solvent system: Ammonia solution, methyl ethyl ketone, and methanol (5:30:65)
4.2.1.2 Analysis
Samples: Impurity solution A, Impurity solution B, Standard solution, and Sample solution
Apply the Samples to a suitable thin-layer chromatographic plate. Develop the chromatograms in the Developing solvent system until the solvent front has moved two-thirds of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate at about 100°-105°. Expose the plate to iodine vapor for about 30 min.
Determine the percentages of each impurity by comparing the intensity of the impurity spots within the Sample solution to those of the main spots from Impurity solution A and Impurity solution B, ignoring any impurity peak less intense than the main spots found in the Impurity solution A and Impurity solution B lanes.
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retardation Factor | Acceptance Criteria, NMT (%) |
| 1-Methylpiperazine | 0.2 | 0.2 |
| 1,4-Dimethylpiperazine | 0.5 | 0.2 |
| Diethylcarbamazine citrate | 1.0 | —▲ (USP 1-Aug-2020) |
4.3 Change to read:
4.3.1 ORGANIC IMPURITIES, PROCEDURE 2
Solution A, Solution B, Mobile phase, and Chromatographic system: Proceed as directed in the Assay.
Citric acid solution: 1 mg/mL of citric acid in Solution BA ▲(USP 1-Aug-2020)
Standard solution: 0.003 mg/mL of USP Diethylcarbamazine Citrate RS in Solution B
Sample solution: 3 mg/mL of Diethylcarbamazine Citrate in Solution B. Filter or centrifuge, and use the clear filtrate or supernatant.
Analysis
Samples: Citric acid solution.▲USP 1-Aug-2020) Standard solution, and Sample solution
Calculate the percentage of each individual impurity in the portion of Diethylcarbamazine Citrate taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response of each individual impurity from the Sample solution
rS = peak response of diethylcarbamazine citrate from the Standard solution
CS = concentration of USP Diethylcarbamazine Citrate RS in the Standard solution (mg/mL) S
CU = concentration of Diethylcarbamazine Citrate in the Sample solution (mg/mL)
Acceptance criteria: See Table 2. Disregard any peak having a retention time corresponding to that of the main peak from the Citric acid solution and any peak below 0.05%.
Table 2
| Name | Acceptance Criteria, NMT (%) |
| Diethylcarbamazine citrate | — |
| Any individual unspecified impurity | 0.10 |
| Total impurities | 0.5 |
(USP 1-Aug-2020)
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method /: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
USP REFERENCE STANDARDS (11)
USP Diethylcarbamazine Citrate RS


