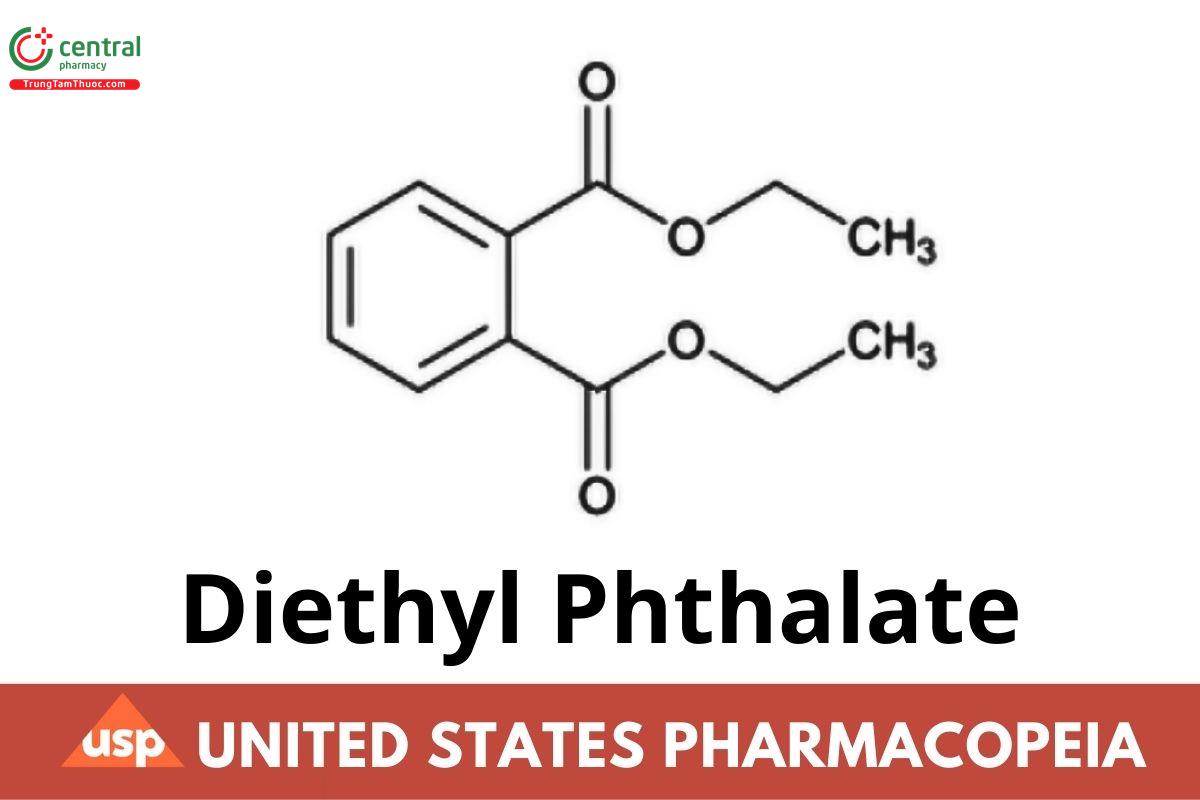
Diethyl Phthalate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Diethyl Phthalate contains NLT 98.0% and NMT 102.0% of diethyl phthalate (C12H14O4), calculated on the anhydrous basis. [Caution-Avoid contact.]
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F.▲ (CN 1-May-2020) On undried specimen
3 ASSAY
Procedure
Sample: 1.5 g
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Residual titration
Titrant: 0.5 N alcoholic potassium hydroxide VS
Back-titrant: 0.5 N hydrochloric acid VS
Endpoint detection: Visual
Analysis: Transfer the Sample to a flask, add 50.0 mL of the Titrant, and boil with a reflux condenser on a water bath for 1 h. Add 20 mL of water, then add phenolphthalein TS. Titrate the excess potassium hydroxide with the Back-titrant. Perform a blank determination. Each mL of 0.5 N potassium hydroxide is equivalent to 55.56 mg of diethyl phthalate (C12H14O4).
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉
Sample: 10 g
Analysis: Gently heat the Sample until the liquid has evaporated, and ignite the residue to constant weight.
Acceptance criteria: NMT 0.02%
5 SPECIFIC TESTS
Specific Gravity 〈841〉: 1.118–1.122 at 20°
Refractive Index 〈831〉: 1.500–1.505 at 20°
Acidity
Sample: 20.0 g
Analysis: Mix the Sample with 50 mL of alcohol that previously has been neutralized to a phenolphthalein endpoint, and titrate with 0.1 N sodium hydroxide VS to a phenolphthalein endpoint.
Acceptance criteria: NMT 0.50 mL is required for neutralization.
Water Determination, Method I〈921〉: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Diethyl Phthalate RS


