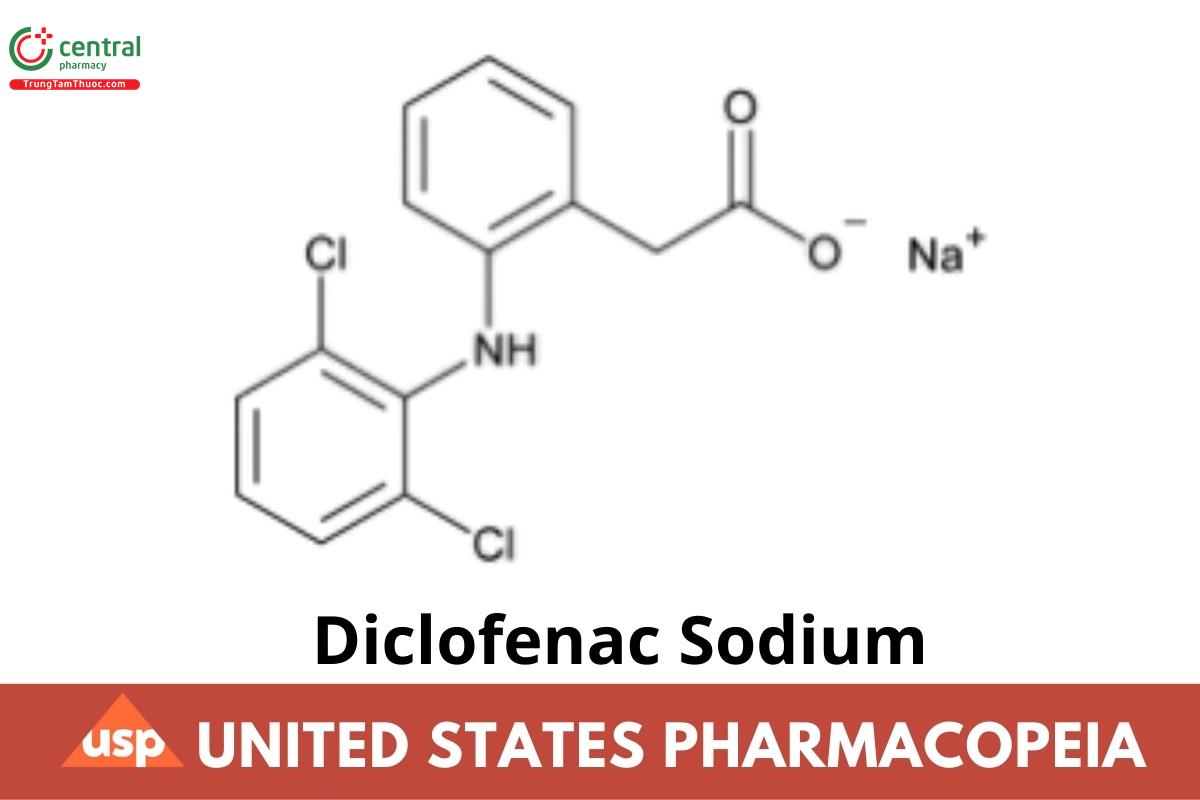
Diclofenac Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Diclofenac Sodium contains NLT 99.0% and NMT 101.0% of diclofenac sodium (C14H10Cl2NNaO2), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The retention time of the diclofenac peak of the Sample solution corresponds to that of the System suitability solution, as obtained in the test for Organic Impurities.
C. The residue obtained by igniting it imparts an intense yellow color to a nonluminous ame.
3 ASSAY
3.1 Procedure
Sample solution: Dissolve about 450 mg of Diclofenac Sodium in 25 mL of glacial acetic acid.
Titrimetric system
Titrant: 0.1 N perchloric acid VS
Analysis: Titrate with Titrant, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 31.81 mg of diclofenac sodium (C14H10Cl2NNaO2).
Acceptance criteria: 99.0%–101.0% on the dried basis
4 IMPURITIES
4.1 Organic Impurities
Solution A: 0.01 M phosphoric acid and 0.01 M monobasic sodium phosphate (1:1). If necessary, adjust with additional portions of the appropriate components to a pH of 2.5 ± 0.2.
Mobile phase: Methanol and Solution A (70:30)
Diluent: Methanol and water (70:30)
System suitability solution: 20 µg/mL of diethyl phthalate, 7.5 µg/mL of USP Diclofenac Related Compound A RS, and 0.75 mg/mL of USP Diclofenac Sodium RS in Diluent
Standard stock solution: 0.75 mg/mL of USP Diclofenac Related Compound A RS in methanol
Standard solution: 1.5 µg/mL of USP Diclofenac Related Compound A RS in Diluent from Standard stock solution Sample solution: 0.75 mg/mL of Diclofenac Sodium in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; packing L7 (end-capped)
Flow rate: 1 mL/min
Injection volume: 10 µL
Run time: 2.5 times the retention time of diclofenac
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for diethyl phthalate, diclofenac related compound A, and diclofenac are 0.5, 0.6, and 1.0, respectively.] Suitability requirements
Resolution: NLT 2.2 between diethyl phthalate and diclofenac related compound A; NLT 6.5 between diclofenac related compound A and diclofenac, System suitability solution
Relative standard deviation: NMT 5%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of diclofenac related compound A in the portion of Diclofenac Sodium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of diclofenac related compound A from the Sample solution
rs = peak response of diclofenac related compound A from the Standard solution
Cs = concentration of USP Diclofenac Related Compound A RS in the Standard solution (mg/mL)
Cu = concentration of Diclofenac Sodium in the Sample solution (mg/mL)
Calculate the percentage of each other impurity in the portion of Diclofenac Sodium taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of each individual impurity from the Sample solution
rs = peak response of diclofenac related compound A from the Standard solution
Cs = concentration of USP Diclofenac Related Compound A RS in the Standard solution (mg/mL)
Cu = concentration of Diclofenac Sodium in the Sample solution (mg/mL)
Acceptance criteria
Diclofenac related compound A: NMT 0.2%
Each other individual impurity: NMT 0.2%
Total impurities: NMT 0.5%
5 SPECIFIC TESTS
5.1 Color of Solution:
A solution (1 in 20) of Diclofenac Sodium in methanol is colorless to faintly yellow, and the absorbance of the solution, determined in a 1-cm cell at 440 nm, is NMT 0.050, methanol being used as the blank.
5.2 Clarity of Solution:
The solution prepared as directed under Color of Solution is not signicantly less clear than an equal volume of methanol contained in a similar vessel and examined similarly.
5.3 pH 〈791〉
Sample solution: A solution (1 in 100)
Acceptance criteria: 7.0–8.5
5.4 Loss on Drying 〈731〉
Analysis: Dry at 105°–110° for 3 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
6.1 Packaging and Storage:
Preserve in tight, light-resistant containers.
6.2 USP Reference Standards 〈11〉
USP Diclofenac Sodium RS
USP Diclofenac Related Compound A RS
N-(2,6-Dichlorophenyl)indolin-2-one.
C14H9Cl2NO 278.14


