Dibucaine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
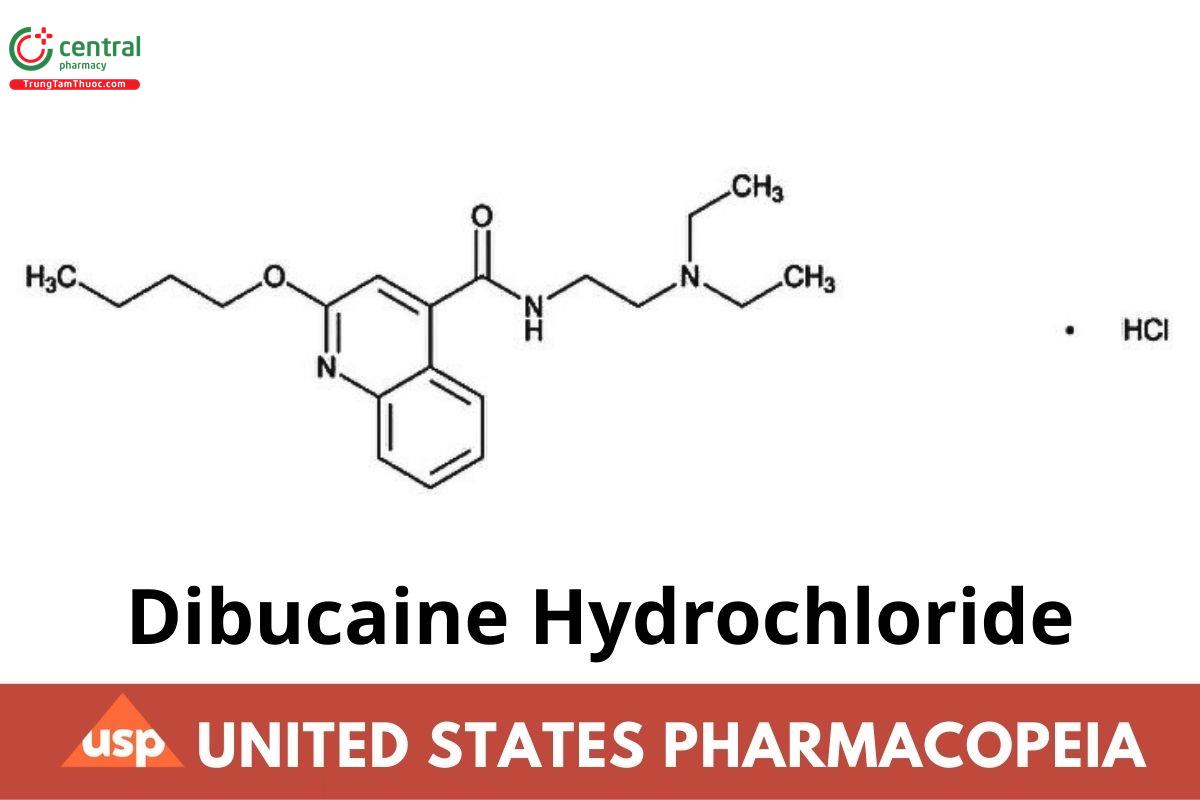
C₂₀H₂₉N₃O₂·HCl 379.92
4-Quinolinecarboxamide, 2-butoxy-N-[2-(diethylamino)ethyl]-, monohydrochloride;
2-Butoxy-N-[2-(diethylamino)ethyl]cinchoninamide monohydrochloride CAS RN®: 61-12-1.
1 DEFINITION
Dibucaine Hydrochloride contains NLT 97.0% and NMT 100.5% of dibucaine hydrochloride (C₂₀H₂₉N₃O₂·HCl), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
Change to read:
C. Identification Tests-General 〈191〉, Chemical Identification Tests, Chloride: Meets the requirements when tested as specified for amine hydrochlorides
3 ASSAY
Change to read:
3.1 Procedure
Mobile phase: Dissolve 1.20 g of sodium dodecyl sulfate, 0.20 g of sodium acetate, and 2.0 mL of triethylamine in 300 mL of water. Adjust with glacial acetic acid to a pH of 5.6. Add 700 mL of methanol, mix, and pass through a filter of 0.5-µm or finer pore size.
Diluent: Methanol and water (70:30)
Standard solution: 1 mg/mL of USP Dibucaine Hydrochloride RS in Diluent. Pass through a filter of 0.5-µm or finer pore size.
Sample solution: 1 mg/mL of Dibucaine Hydrochloride in Diluent. Pass through a filter of 0.5-µm or finer pore size.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 254 nm
- Column: 3.9-mm × 30-cm; 10-µm packing L1
- Flow rate: 1.5 mL/min
- Injection volume: 10 µL
- Run time: NLT 2.5 times the retention time of dibucaine
System suitability
- Sample: Standard solution
- Suitability requirements
- Column efficiency: NLT 1500 theoretical plates
- Tailing factor: NMT 3.0
- Relative standard deviation: NMT 2%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of dibucaine hydrochloride (C₂₀H₂₉N₃O₂·HCl) in the portion of Dibucaine Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of dibucaine from the Sample solution
rₛ = peak response of dibucaine from the Standard solution
Cₛ = concentration of USP Dibucaine Hydrochloride RS in the Standard solution (mg/mL)
Cᵤ = concentration of Dibucaine Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–100.5% on the dried basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
4.2 Organic Impurities
Standard solution: 40 mg/mL of USP Dibucaine Hydrochloride RS in chloroform
Sample solution: 40 mg/mL of Dibucaine Hydrochloride in chloroform
Comparison solution A: 40 µg/mL of USP Dibucaine Hydrochloride RS from the Standard solution in chloroform (0.1%)
Comparison solution B: 120 µg/mL of USP Dibucaine Hydrochloride RS from the Standard solution in chloroform (0.3%)
Comparison solution C: 200 µg/mL of USP Dibucaine Hydrochloride RS from the Standard solution in chloroform (0.5%)
Chromatographic system
- (See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
- Mode: TLC
- Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
- Application volume: 5 µL
- Developing solvent system: Toluene, acetone, methanol, and ammonium hydroxide (50:30:5:1)
- Spray reagent: 5 mg/mL of potassium dichromate in 7 N sulfuric acid TS
Analysis
Samples: Standard solution, Sample solution, and each Comparison solution
Proceed as directed in the general chapter. Develop the chromatogram in the Developing solvent system until the solvent front has moved three-fourths of the length of the plate. Remove the plate from the chamber, mark the solvent front, and air-dry. Spray the plate heavily with Spray reagent. Place the plate in an oven at 140° for 10 min, and view under short-wavelength UV light.
Acceptance criteria: The principal spot from the Sample solution corresponds in Rf value, color, and intensity to that from the Standard solution; the sum of the intensities of any secondary spots, if present in the Sample solution, is NMT 1.0%, and the intensity of any secondary spot does not exceed 0.5% of that of the principal spot from the Standard solution on the basis of comparison with the spots from each Comparison solution.
5 SPECIFIC TESTS
5.1 Loss on Drying 〈731〉
Analysis: Dry at 80° for 5 h.
Acceptance criteria: NMT 2.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
USP Reference Standards 〈11〉
USP Dibucaine Hydrochloride RS


