Diatrizoate Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
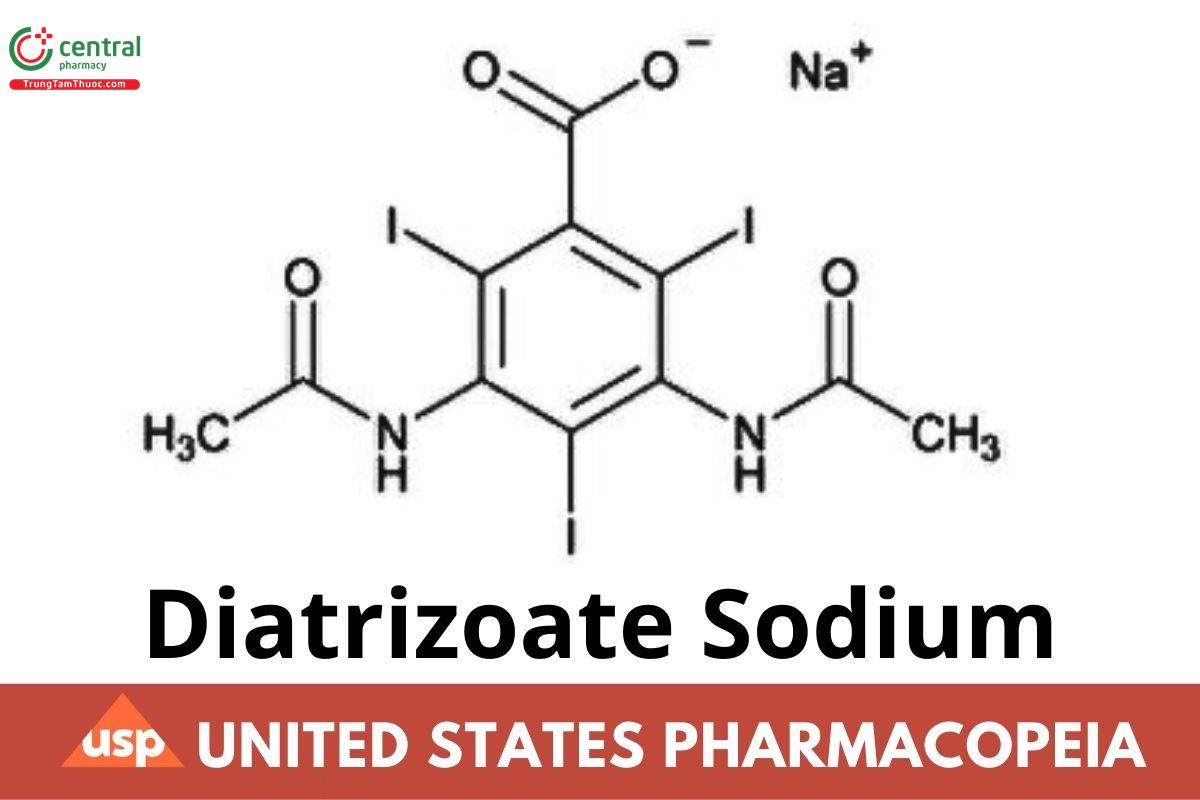
C₁₁H₈I₃N₂NaO₄ 635.90
Benzoic acid, 3,5-bis(acetylamino)-2,4,6-triiodo-, monosodium salt;
Monosodium 3,5-diacetamido-2,4,6-triiodobenzoate CAS RN®: 737-31-5; UNII: V5403H8VG7.
1 DEFINITION
Diatrizoate Sodium contains NLT 98.0% and NMT 102.0% of diatrizoate sodium (C₁₁H₈I₃N₂NaO₄), calculated on the anhydrous basis.
2 IDENTIFICATION
2.1 A. Thin-Layer Chromatographic Identification Test 〈201〉
Solution A: Sodium hydroxide in methanol (0.8 in 1000)
Standard solution: 1 mg/mL of USP Diatrizoic Acid RS in Solution A
Sample solution: 1 mg/mL of Diatrizoate Sodium in Solution A
Developing solvent system: Methanol, chloroform, and ammonium hydroxide (10:20:2)
Analysis
Samples: Standard solution and Sample solution
Locate the spots using short-wavelength UV light.
Acceptance criteria: Meets the requirements
2.2 B.
A 5-mg/mL solution in water imparts an intense yellow color to a nonluminous flame confirming the presence of sodium.
3 ASSAY
3.1 Procedure
Sample solution: Transfer 300 mg of Diatrizoate Sodium to a glass-stoppered, 125-mL conical flask, add 30 mL of 1.25 N sodium hydroxide and 500 mg of powdered zinc, connect the flask to a reflux condenser, and reflux the mixture for 1 h. Cool the flask to room temperature, rinse the condenser with 20 mL of water, disconnect the flask from the condenser, and filter the mixture. Rinse the flask and filter thoroughly, adding the rinsings to the filtrate. Add 5 mL of glacial acetic acid and 1 mL of tetrabromophenolphthalein ethyl ester TS.
Titrimetric system
- (See Titrimetry 〈541〉.)
- Mode: Direct titration
- Titrant: 0.05 N silver nitrate VS
- Endpoint detection: Visual
Analysis: Titrate the Sample solution with the Titrant until the yellow precipitate just turns green.
Calculate the percentage of diatrizoate sodium (C₁₁H₈I₃N₂NaO₄) in the portion of Diatrizoate Sodium taken:
Result = [(V × N × F)/W] × 100
V = sample Titrant volume (mL)
N = Titrant normality (mEq/mL)
F = equivalent weight of diatrizoate sodium, 212 mg/mEq
W = weight of Diatrizoate Sodium (mg)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Change to read:
4.1 Limit of Free Aromatic Amine
Solution A: 1 mg/mL of N-(1-naphthyl)ethylenediamine dihydrochloride in a mixture of propylene glycol and water (70:30)
Standard stock solution: 0.5 mg/mL of USP Diatrizoic Acid Related Compound A RS prepared as follows. Transfer 5 mg of USP Diatrizoic Acid Related Compound A RS to a 10-mL volumetric flask. Add 0.2 mL of 0.1 N sodium hydroxide. Dilute with water to volume.
Standard solution: Transfer 4 mL of water, 10 mL of 0.1 N sodium hydroxide, and 1.0 mL of Standard stock solution to a 50-mL volumetric flask.
Sample solution: Transfer 1.0 g of Diatrizoate Sodium to a 50-mL volumetric flask, and add 5 mL of water and 10 mL of 0.1 N sodium hydroxide.
Blank solution: Transfer 5 mL of water and 10 mL of 0.1 N sodium hydroxide to a 50-mL volumetric flask.
Instrumental conditions
- Mode: Vis
- Analytical wavelength: 465 nm
- Cell: 1 cm
Analysis
Samples: Standard solution, Sample solution, and Blank solution
Treat the Samples as follows. Add 25 mL of dimethylsulfoxide. Place the flask in an ice bath for 5 min. Add 2 mL of hydrochloric acid, and allow to stand in the ice bath for 5 min. Add 2.0 mL of sodium nitrite solution (20 mg/mL), and allow to stand in the ice bath for 5 min. Remove the flask from the ice bath, add 1.0 mL of sulfamic acid solution (80 mg/mL), and allow to stand in the ice bath for 5 min. [Caution-Considerable pressure is produced.] Add 2.0 mL of Solution A. Remove the flasks from the ice bath and allow to stand in a water bath at room temperature. Shake occasionally, releasing the pressure by loosening the stopper. Dilute with water to volume. Within 5 min, measure the absorbances of the Standard solution and Sample solution against the blank.
Acceptance criteria: The absorbance of the Sample solution is NMT that of the Standard solution (NMT 0.05% of free aromatic amine).
5 SPECIFIC TESTS
5.1 Water Determination 〈921〉, Method I: NMT 10.0%
5.2 Limit of Iodine and Iodide
Standard solution: Dissolve 0.5 mg of potassium iodide in 24 mL of water in a 50-mL stoppered centrifuge tube.
Sample solution: Transfer 2.0 g to a 50-mL stoppered centrifuge tube, dilute with water to 24 mL, and shake to dissolve.
Analysis
Samples: Standard solution and Sample solution
Treat the Samples as follows. Add 5 mL of toluene and 5 mL of 2 N sulfuric acid, shake, and centrifuge. The toluene layer shows no red color. Add 1 mL of sodium nitrite solution (1 in 50), shake, and centrifuge.
Acceptance criteria
- The Sample solution does not show any red color in the toluene layer prior to addition of the sodium nitrite solution.
- The red color of the toluene layer in the Sample solution after the addition of sodium nitrite solution is not darker than the Standard solution (NMT 0.02% of iodide).
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
USP Reference Standards 〈11〉
- USP Diatrizoic Acid RS
- USP Diatrizoic Acid Related Compound A RS
5-Acetamido-3-amino-2,4,6-triiodobenzoic acid.
C₉H₇I₃N₂O₃ 571.88


