Dextrose
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
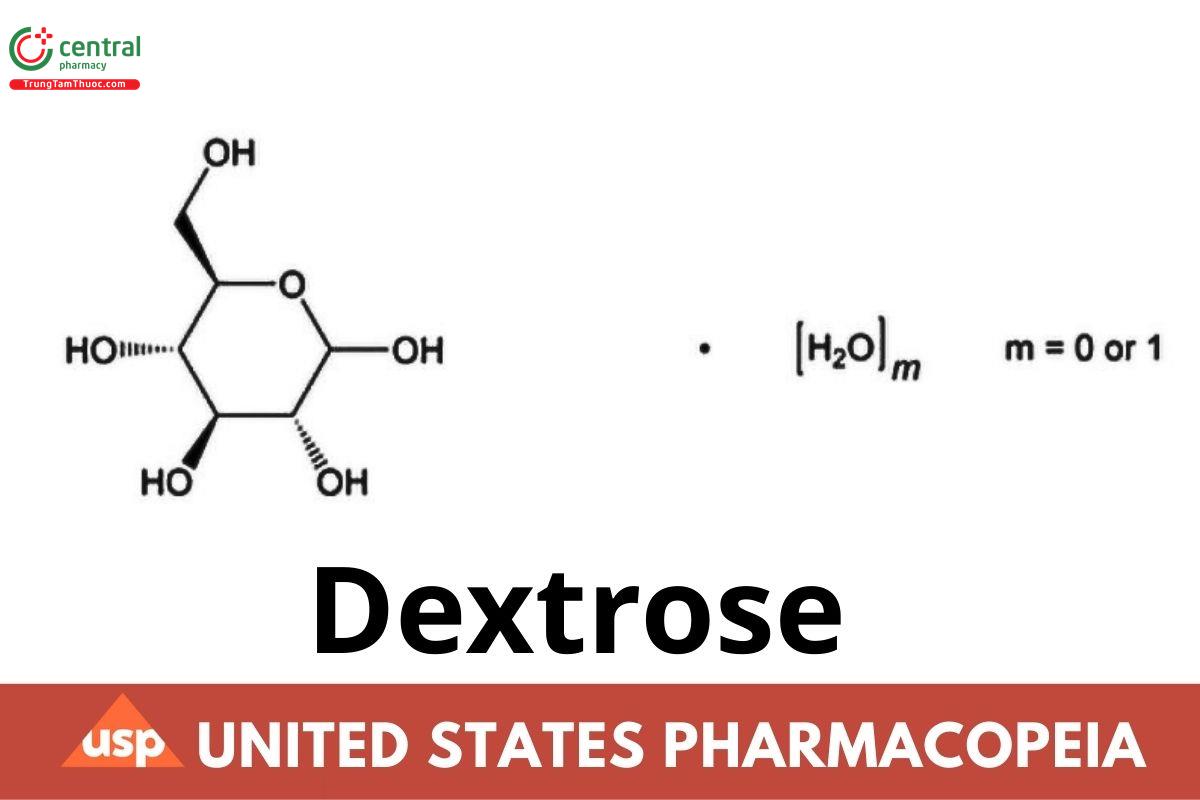
C₆H₁₂O₆·H₂O 198.17
C₆H₁₂O₆ 180.16
D-Glucose monohydrate CAS RN®: 77938-63-7.
D-Glucose anhydrous CAS RN®: 50-99-7.
1 DEFINITION
Dextrose is (+)-D-glucopyranose and is derived from starch. It contains one molecule of water of hydration or is anhydrous. It contains NLT 97.5% and NMT 102.0% of dextrose, calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
2.1 A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A
Sample: Dry at 70° under vacuum for at least 2 h to constant weight.
Acceptance criteria: Meets the requirements
2.2 B.
Analysis: Examine the chromatograms from the Assay.
Acceptance criteria: The principal peak from the Sample solution is similar in retention time and size to the principal peak from Standard solution A.
2.3 C. Water Determination 〈921〉, Method I
Samples
- Anhydrous: 0.50 g
- Monohydrate: 0.25 g
Acceptance criteria
- Anhydrous: NMT 1.0%
- Monohydrate: 7.5%–9.5%
3 ASSAY
3.1 Procedure
Mobile phase: Water
System suitability solution: Dissolve 5 mg of USP Maltose Monohydrate RS, 5 mg of USP Maltotriose RS, and 5 mg of USP Fructose RS in water and dilute with water to 50.0 mL.
Standard solution A: 30 mg/mL of USP Dextrose RS
Sample solution: 30 mg/mL, determined on the anhydrous basis
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: Refractive index
- Column: 7.8-mm × 30-cm; 9-µm packing L191
- Temperatures
- Column: 85 ± 1°
- Detector: 40°
- Flow rate: 0.3 mL/min
- Injection volume: 20 µL
- Run time: About 1.5 times the retention time of dextrose
System suitability
- Sample: System suitability solution
- [Note-The relative retention times for maltotriose, maltose, isomaltose, dextrose, and fructose are 0.7, 0.8, 0.8, 1.0, and 1.3, respectively. The retention time for dextrose is about 21 min.]
- Suitability requirement
- Resolution: NLT 1.3 between maltotriose and maltose
Analysis
Samples: Standard solution A and Sample solution
Calculate the percentage, on the anhydrous basis, of dextrose (C₆H₁₂O₆) in the portion of Dextrose taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of dextrose from the Sample solution
rₛ = peak response of dextrose from Standard solution A
Cₛ = concentration of USP Dextrose RS in Standard solution A (mg/mL)
Cᵤ = concentration of the Sample solution on the anhydrous basis (mg/mL)
Acceptance criteria: 97.5%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 Related Substances
Mobile phase, System suitability solution, Standard solution A, and Chromatographic system: Proceed as directed in the Assay.
Sample solution: 30 mg/mL, determined on the anhydrous basis
Standard solution B: Dilute 1.0 mL of the Sample solution with water to 250.0 mL.
Standard solution C: Dilute 25.0 mL of Standard solution B with water to 200.0 mL.
System suitability
- Sample: System suitability solution
- [Note-The relative retention times for maltotriose, maltose, isomaltose, dextrose, and fructose are 0.7, 0.8, 0.8, 1.0, and 1.3, respectively.
- The retention time for dextrose is about 21 min.]
- Suitability requirement
- Resolution: NLT 1.3 between maltotriose and maltose
Analysis
Samples: Standard solution A, Sample solution, Standard solution B, and Standard solution C
Disregard any peak with an area less than the principal peak from Standard solution C (0.05%).
Acceptance criteria
- For maltose and isomaltose: NMT 0.4%. The sum is NMT the area of the principal peak from Standard solution B.
- For maltotriose: NMT 0.2%. NMT 0.5 times the area of the principal peak from Standard solution B.
- For fructose: NMT 0.15%. NMT 3 times the area of the principal peak from Standard solution C.
- Unspecified: NMT 0.10%. NMT twice the area of the principal peak from Standard solution C.
- Total impurities: NMT 0.5%. NMT 1.25 times the area of the principal peak from Standard solution B.
5 SPECIFIC TESTS
5.1 Color and Clarity of Solution
Reference solution: To 2.5 mL of cobaltous chloride CS, 6.0 mL of ferric chloride CS, and 1.0 mL of cupric sulfate CS add hydrochloric acid [10 g/L of hydrogen chloride (HCl)] to make 1000.0 mL.
Hydrazine sulfate solution: Dissolve 1.0 g of hydrazine sulfate in water and dilute to 100.0 mL. Allow to stand for 4–6 h.
Hexamethylenetetramine solution: In a 100-mL ground-glass-stoppered flask, dissolve 2.5 g of hexamethylenetetramine in 25.0 mL of water.
Primary opalescent suspension: To the Hexamethylenetetramine solution in the flask add 25.0 mL of the Hydrazine sulfate solution. Mix and allow to stand for 24 h. This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use.
Standard of opalescence: Dilute 15.0 mL of the Primary opalescent suspension with water to 1000.0 mL. This suspension is freshly prepared and may be stored for up to 24 h.
Reference suspension: To 5.0 mL of Standard of opalescence add 95.0 mL of water. Mix and shake before use.
Sample solution: Dissolve 10.0 g in 15 mL of water using a bath of boiling water. Allow to cool.
Analysis: Make the comparison by viewing the solutions downward in matched color–comparison tubes against a white surface (see Visual Comparison 〈630〉).
Acceptance criteria: The Sample solution is clear (its clarity is the same as that of water or its opalescence is not more pronounced than that of the Reference suspension) and not more intensely colored than the Reference solution.
5.2 Conductivity
Sample solution: Dissolve 20.0 g in carbon dioxide-free water prepared from distilled water and dilute with the same solvent to 100.0 mL.
Analysis: Measure the conductivity of the solution while gently stirring with a magnetic stirrer.
Acceptance criteria: NMT 20 µS/cm at 25°
5.3 Dextrin
Sample: 1 g, finely powdered
Analysis: Reflux the Sample with 20 mL of alcohol.
Acceptance criteria: The Sample dissolves completely.
5.4 Soluble Starch, Sulfites
Sample solution: Dissolve the Dextrose sample (6.7 g of anhydrous or 7.4 g of monohydrate) in 15 mL of water using a bath of boiling water. Allow to cool.
Analysis: To the Sample solution add 25 µL of iodine TS.
Acceptance criteria: The resulting solution is yellow (NMT 15 ppm).
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
Labeling: Label to indicate whether it is hydrous or anhydrous.
USP Reference Standards 〈11〉
- USP Dextrose RS
- USP Fructose RS
- USP Maltose Monohydrate RS
- USP Maltotriose RS
1 Aminex HPX-87C from Biorad is suitable.


