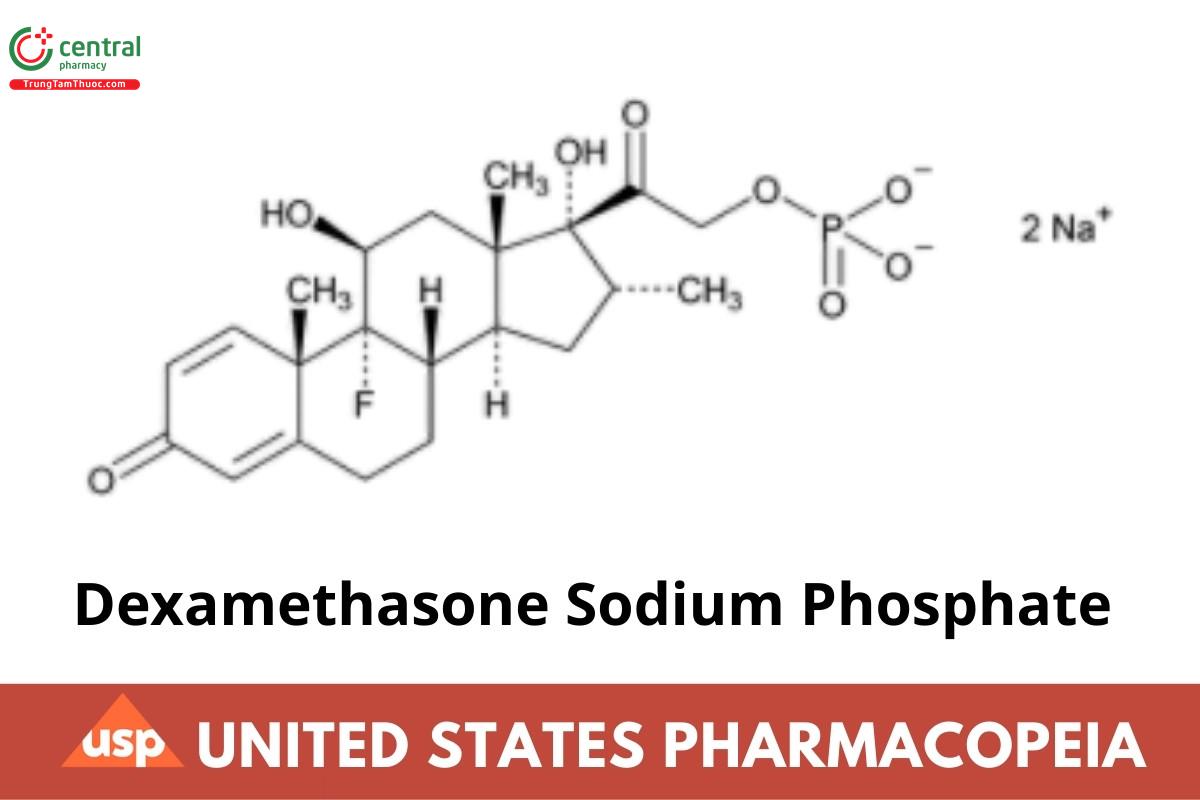
Dexamethasone Sodium Phosphate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Dexamethasone Sodium Phosphate contains NLT 97.0% and NMT 102.0% of dexamethasone sodium phosphate (C22H28FNa2O8P), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020) : If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the Reference Standard separately in a minimum of alcohol, evaporate on a water bath to dryness, and repeat the test on the residues.
B. Identification Tests—General, Phosphate〈191〉: The residue from its ignition meets the requirements.
C. Identification Tests—General, Sodium〈191〉: The residue from its ignition meets the requirements.
3 ASSAY
3.1 Procedure
Mobile phase: Mix 520 mL of water with 2 mL of phosphoric acid. Bring the temperature to 20°, and adjust with sodium hydroxide to a pH of 2.6. Mix this solution with 36 mL of tetrahydrofuran and 364 mL of methanol.
System suitability stock solution: 0.02 mg/mL each of USP Dexamethasone Sodium Phosphate RS and USP Dexamethasone RS, prepared as follows. Dissolve 2 mg of each compound in 2 mL of tetrahydrofuran, and dilute with Mobile phase to 100 mL.
System suitability solution: 2 µg/mL each of USP Dexamethasone Sodium Phosphate RS and USP Dexamethasone RS in Mobile phase from the System suitability stock solution
Standard solution: 0.06 mg/mL of USP Dexamethasone Sodium Phosphate RS in Mobile phase
Sample solution: 0.06 mg/mL of Dexamethasone Sodium Phosphate in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 7-µm packing L1
Flow rate: 1.5 mL/min
Injection volume: 20 µL
Run time: 3 times the retention time of the dexamethasone sodium phosphate peak
System suitability
[Note—The relative retention times of the dexamethasone sodium phosphate and dexamethasone peaks are 1.0 and 2.0, respectively.] Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 6.0 between dexamethasone sodium phosphate and dexamethasone peaks, System suitability solution Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of dexamethasone sodium phosphate (C22H28FNa2O8P) in the portion of Dexamethasone Sodium Phosphate taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Dexamethasone Sodium Phosphate RS in the Standard solution (mg/mL)
Cu = nominal concentration of Dexamethasone Sodium Phosphate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
4.1 Limit of Phosphate Ions
Solution A: 50 mg/mL of ammonium molybdate in 1 N sulfuric acid
Solution B: Dissolve 350 mg of p-methylaminophenol sulfate in 50 mL of water, add 20 g of sodium bisulte, mix to dissolve, and dilute with water to 100 mL.
Standard stock solution: 0.14 mg/mL of dried monobasic potassium phosphate in water. This solution contains the equivalent of 0.10 mg/mL of phosphate (PO4) ion.
Standard solution: In a 25-mL volumetric ask, mix 5.0 mL of Standard stock solution, 10 mL of water, and 5 mL of 2 N sulfuric acid. Add 1 mL each of Solution A and Solution B, dilute with water to volume, and allow to stand at room temperature for 30 min. Prepare concomitantly with the Sample solution.
Sample solution: In a 25-mL volumetric ask, dissolve 50 mg of Dexamethasone Sodium Phosphate in a mixture of 10 mL of water and 5 mL of 2 N sulfuric acid, by warming if necessary. Add 1 mL each of Solution A and Solution B, dilute with water to volume, and allow to stand at room temperature for 30 min.
Instrumental conditions
(See Ultraviolet-Visible Spectroscopy 〈857〉.)
Mode: Visible
Analytical wavelength: 730 nm
Cell: 1 cm
Blank: Water
Analysis
Samples: Standard solution and Sample solution
Acceptance criteria: The absorbance of the Sample solution is NMT that of the Standard solution. The limit is 1.0% of phosphate (PO4).
4.2 Limit of Alcohol
Internal standard solution: 0.4 mg/mL of acetonitrile, prepared as follows. Dilute 5.0 mL of USP Alcohol Determination–Acetonitrile RS with water to 200.0 mL.
Standard stock solution: 0.20 mg/mL of alcohol, prepared as follows. Dilute 25.0 mL of USP Alcohol Determination–Alcohol RS with water to 2000.0 mL.
Standard solution: 0.08 mg/mL of alcohol, prepared as follows. Dilute 10.0 mL of the Standard stock solution and 5.0 mL of the Internal standard solution with water to 25.0 mL.
Sample solution: 5.0 mg/mL of Dexamethasone Sodium Phosphate, prepared as follows. Transfer 125 mg of Dexamethasone Sodium Phosphate to a 25-mL volumetric ask, add 5.0 mL of the Internal standard solution, and dilute with water to volume. Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.53-mm × 30-m capillary coated with 3-µm lm of G43
Carrier gas: Hydrogen
Linear velocity: 36 cm/s
Split ratio: 2:1
Temperatures
Injection port: 210°
Detector: 280°
Column: See Table 1.
Table 1
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 50 | _ | 50 | 5 |
| 50 | 50 | 200 | 4 |
Injection volume: 0.4 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 2.0 between alcohol and acetonitrile
Relative standard deviation: NMT 3.0% for alcohol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of alcohol in the portion of Dexamethasone Sodium Phosphate taken:
Result = (Ru/Rs) × (Cs/Cu) × 100
Ru = peak response ratio of the alcohol to the internal standard from the Sample solution
Rs = peak response ratio of the alcohol to the internal standard from the Standard solution
Cs = concentration of alcohol in the Standard solution (mg/mL)
Cu = concentration of Dexamethasone Sodium Phosphate in the Sample solution (mg/mL)
Acceptance criteria: NMT 1.5%
4.3 Organic Impurities
Buffer: 7.0 g/L of ammonium acetate in water
Solution A: Mix 300 mL of Buffer and 350 mL of water, adjust with 5 M acetic acid to a pH of 3.8, and then add 350 mL of methanol. Solution B: Adjust 300 mL of Buffer with 5 M acetic acid to a pH of 4.0, and then add 700 mL of methanol.
Mobile phase: See Table 2.
Table 2
Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 3.5 | 90 | 10 |
| 23.5 | 60 | 40 |
| 34.5 | 5 | 95 |
| 50 | 5 | 95 |
System suitability solution: 0.02 mg/mL each of USP Dexamethasone Sodium Phosphate RS and USP Betamethasone Sodium Phosphate RS in Solution A
Standard solution: 1 µg/mL of USP Dexamethasone Sodium Phosphate RS in Solution A
Sample solution: 1 mg/mL of Dexamethasone Sodium Phosphate in Solution A
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 12.5-cm; 5-µm packing L7
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 2.0 between dexamethasone sodium phosphate and betamethasone sodium phosphate peaks, System suitability solution Relative standard deviation: NMT 5.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Dexamethasone Sodium Phosphate taken:
Result = (ru/rs) × (Cs/Cu) × (1/F) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of dexamethasone phosphate from the Standard solution
Cs = concentration of USP Dexamethasone Sodium Phosphate RS in the Standard solution (µg/mL)
Cu = concentration of Dexamethasone Sodium Phosphate in the Sample solution (µg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. Disregard any peak below 0.05%.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| 16(17)a-Homodexamethasone sodium phosphate | 0.5 | 1.0 | 0.2 |
| 16(17)a-Homobetamethasone sodium phosphate | 0.6 | 1.0 | 0.2 |
16(17)a-17R Homodexamethasone sodium phosphate | 0.8 | 1.0 | 0.2 |
13(17)a-Homodexamethasone sodium phosphate | 0.92 | 1.0 | 0.2 |
Betamethasone sodium phosphate | 0.95 | 1.0 | 0.2 |
Dexamethasone sodium phosphate | 1.00 | _ | _ |
Dexamethasone ethyl estere | 1.20 | 1.0 | 0.3 |
Dexamethasone | 1.37 | 1.2 | 0.5 |
| Fluoroandrostadiene carboxylic acid | 1.41 | 1.0 | 0.3 |
Dexamethasone sodium phosphate diester | 2.10 | 1.0 | 0.1 |
Any other individual unspecied impurity | _ | 1.0 | 0.10 |
| Total impurities | _ | _ | 1.0 |
a 9-Fluoro-11β,17,21-trihydroxy-16α-methyl-16(17)a-homopregna-1,4-diene-3,16a,20-trione 21-(dihydrogen phosphate) disodium salt.
b 9-Fluoro-11β,17,21-trihydroxy-16β-methyl-16(17)a-homopregna-1,4-diene-3,16a,20-trione 21-(dihydrogen phosphate) disodium salt.
c 9-Fluoro-11β,17a,21-trihydroxy-16β-methyl-16(17)a-homopregna-1,4-diene-3,16a,20-trione 21-(dihydrogen phosphate) disodium salt.
d 9-Fluoro-11β,17,21-trihydroxy-16α-methyl-13(17)a-homopregna-1,4-diene-3,13a,20-trione 21-(dihydrogen phosphate) disodium salt.
e Ethyl 11β,17α-dihydroxy-9-uoro-16α-methylandrostane-1,4-diene-3-one-17-ylcarboxylate.
f 9-Fluoro-11β,17α-dihydroxy-16α-methylandrosta-1,4-diene-3-one-17β-carboxylic acid.
g Sodium bis[9-uoro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 21-yl]phosphate.
5 SPECIFIC TESTS
5.1 Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL in water
Acceptance criteria: +74° to +82°, calculated on the anhydrous and solvent-free basis
5.2 pH 〈791〉
Sample solution: 10 mg/mL
Acceptance criteria: 7.5–10.5
5.3 Water Determination, Method I〈921〉
Acceptance criteria: NMT 10.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
USP Reference Standards 〈11〉
USP Alcohol Determination–Acetonitrile RS
USP Alcohol Determination–Alcohol RS
USP Betamethasone Sodium Phosphate RS
USP Dexamethasone RS
USP Dexamethasone Sodium Phosphate RS


