Desonide
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
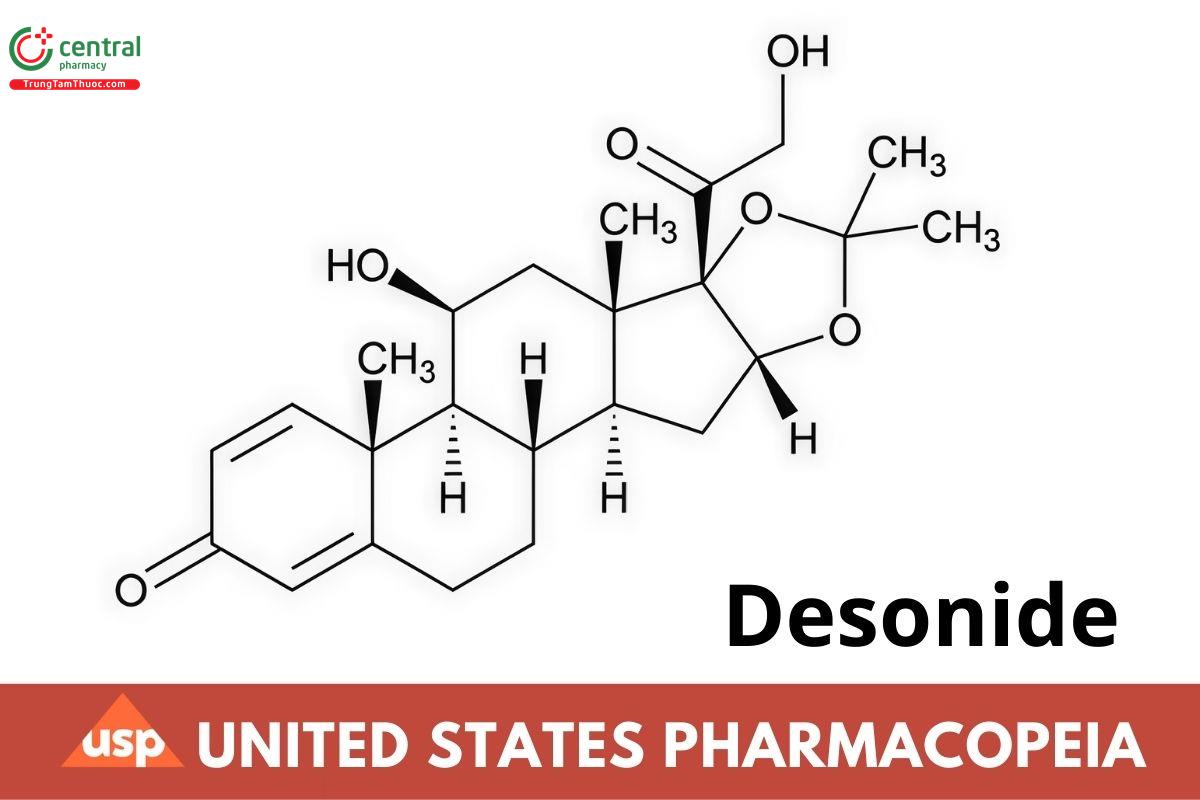
C24H32O6 416.51
Pregna-1,4-diene-3,20-dione, 11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy))-, (118,16α)-;
118,16,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone CAS RN®: 638-94-8; UNII: J280872010.
1 DEFINITION
Desonide contains NLT 98.0% and NMT 102.0% of the labeled amount of desonide (C24H32O6), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K ▲(CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Use low-actinic glassware for all solutions containing desonide.
Solution A: 0.1% Phosphoric acid in water
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 70 | 30 |
| 4 | 68 | 32 |
| 5 | 68 | 32 |
| 9 | 65 | 35 |
| 13 | 30 | 70 |
| 16 | 30 | 70 |
Diluent: Solution A and acetonitrile (60:40)
System suitability solution: 0.7 mg/mL of USP Desonide Impurities Mixture RS in Diluent
Standard solution: 0.7 mg/mL of USP Desonide RS in Diluent
Sample solution: 0.7 mg/mL of Desonide in Diluent
3.1.1 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 15-cm; 2.6-µm packing L7
3.1.2 Temperatures
Column: 20° (15°-22° was shown to be acceptable)
Autosampler: 20°
Flow rate: 1 mL/min
Injection volume: 5 µL
3.1.3 System suitability
Samples: System suitability solution and Standard solution
3.1.4 Suitability requirements
Resolution: NLT 2.0 between desonide glyoxal and deoxyprednisolone-16-ene; NLT 2.0 between desonide and dihydrodesonide, System suitability solution
Relative standard deviation: NMT 0.73%, Standard solution
3.1.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of desonide (C24H32O6) in the portion of Desonide taken:
Result = (rU/rS) x (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution s
CS = concentration of USP Desonide RS in the Standard solution (mg/mL)
CU = concentration of Desonide in the Sample solution ( mg/mL)
Acceptance criteria: 98.0%-102.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281): NMT 0.10%
4.2 ORGANIC IMPURITIES
Solution A, Solution B, Mobile phase, Diluent, System suitability solution, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Sensitivity solution: 0.28 µg/mL of USP Desonide RS in Diluent from the Standard solution
4.2.1 System suitability
Samples: System suitability solution and Sensitivity solution
4.2.2 Suitability requirements
Resolution: NLT 2.0 between desonide glyoxal and deoxyprednisolone-16-ene; NLT 2.0 between desonide and dihydrodesonide, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
4.2.3 Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Desonide taken:
Result = (rU/rT) x (1/F) x 100
rU = peak area of each impurity from the Sample solution
rT = total peak area from the Sample solution
F = relative response factor (see Table 2)
Acceptance criteria: See Table 2.
[NOTE-Disregard peaks that are less than 0.04% of the desonide peak from the Standard solution.]
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| 16α-Hydroxyprednisolonea | 0.31 | 1.0 | 0.15 |
| Prednisoloneb | 0.48 | 1.0 | 0.15 |
| Desonide glyoxalc | 0.76 | 1.0 | 0.50 |
| Deoxyprednisolone-16-ened | 0.82 | 1.7 | 0.50 |
| Sum of desonide glyoxal and deoxyprednisolone-16-ene | — | — | 0.50 |
| Desonide | 1.00 | — | — |
| Dihydrodesonidee | 1.07 | 0.84 | 0.50 |
| Epoxydesonidef | 1.18 | 1.0 | 0.50 |
| Sum of dihydrodesonide and epoxydesonide | — | — | 0.50 |
| Bromodesonideg | 1.43 | 0.68 | 0.15 |
| Acetyldesonideh | 1.74 | 0.82 | 0.15 |
| Any other impurity | — | 1.0 | 0.10 |
| Total impurities | — | — | 1.0 |
a 11ẞ,16α,17,21-Tetrahydroxy-3,20-dioxopregna-1,4-diene.
b 11ẞ,17,21-Trihydroxy-3,20-dioxopregna-1,4-diene.
c 11ẞ-Hydroxy-16α,17-[(1-methylethylidene)bis(oxy)]-3,20-dioxopregna-1,4-dien-21-al.
d 11ẞ,21-Dihydroxypregna-1,4,16-triene-3,20-dione.
e 11ẞ,21-Dihydroxy-16α, 17-[(1-methylethylidene)bis(oxy)]pregn-4-ene-3,20-dione.
f 11ẞ-Epoxy-21-hydroxy-16α, 17-(1-methylethylidenedioxy)pregna-1,4-diene-3,20-dione.
g 9α-Bromo-113,21-dihydroxy-16α, 17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione.
h 11ẞ-Hydroxy-16α,17-[(1-methylethylidene)bis(oxy)]-3,20-dioxopregna-1,4-dien-21-yl acetate.
5 SPECIFIC TESTS
5.1 LOSS ON DRYING (731)
Sample: 1.0 g
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 0.5%
5.2 OPTICAL ROTATION, Specific Rotation (781S)
Sample: 10 mg/mL in dioxane
Acceptance criteria: +104.0° to +110.0°, calculated on the dried basis
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, and store at controlled room temperature. Protect from light.
USP REFERENCE STANDARDS (11)
USP Desonide RS
USP Desonide Impurities Mixture RS
This is a mixture that contains desonide and may contain about 1% each of 16a-hydroxyprednisolone, Prednisolone, desonide glyoxal, deoxyprednisolone-16-ene, dihydrodesonide, epoxydesonide, bromodesonide, and acetyldesonide.


