Desmopressin Acetate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
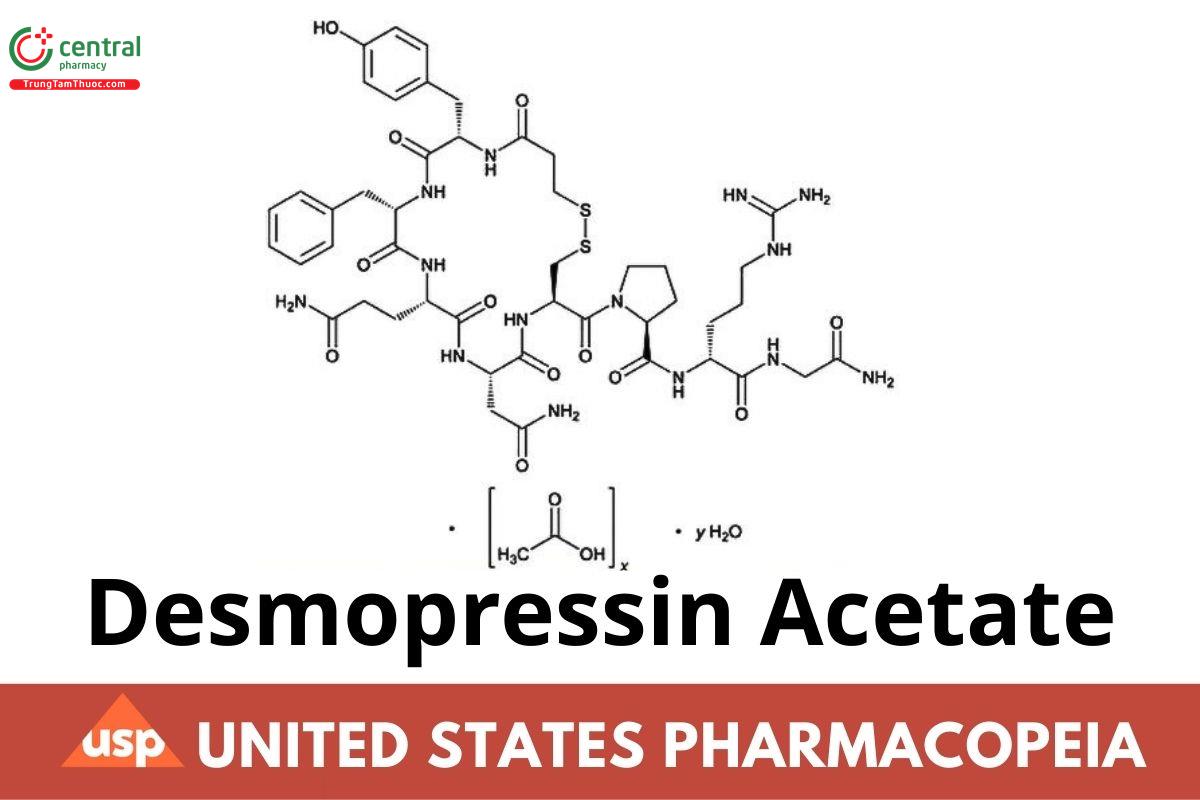
C46H64N14O12S2 · xC2H4O2 · yH2O 1069.22 (anhydrous, free base)
Vasopressin, 1-(3-mercaptopropanoic acid)-8-d-arginine-, acetate (salt) hydrate;
1-(3-Mercaptopropionic acid)-8-d-arginine-vasopressin, acetate (salt) hydrate.
x(acetate), y(water).
Monoacetate trihydrate CAS RN®: 62357-86-2; UNII: XB13HYU18U.
Monoacetate anhydrous CAS RN®: 62288-83-9; UNII: 1K12647SFC.
1 DEFINITION
Desmopressin Acetate is a synthetic octapeptide hormone having the property of antidiuresis. It is a synthetic analog of Vasopressin. It contains NLT 95.0% and NMT 105.0% of desmopressin (C46H64N14O12S2), calculated on the anhydrous, acetic acid-free basis.
2 IDENTIFICATION
2.1 A. The monoisotopic mass by Mass Spectrometry 〈736〉 is 1068.4 ± 0.5 mass units.
2.2 B.
Buffer solution, Mobile phase, Standard solution, and Sample solution: Prepare as directed in the Assay.
Identity sample solution: 10 µg/mL each of USP Desmopressin Acetate RS and Desmopressin Acetate in Mobile phase
Acceptance criteria: The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. The major peaks of the Identity sample solution coelute.
3 ASSAY
Change to read:
3.1 Procedure
Buffer solution: Dissolve 3.4 g of monobasic potassium phosphate and 2.0 g of sodium 1-heptanesulfonic acid in 1000 mL of water. Adjust with phosphoric acid or sodium hydroxide to a pH of 4.50 ± 0.05, as needed. Pass through a filter of 0.45-µm pore size.
Mobile phase: Mix acetonitrile and Buffer solution (22:78), and degas. Make adjustments, if necessary (see Chromatography (621). System Suitability). [NOTE-The retention time of desmopressin is very sensitive to the composition of the Mobile phase.]
Standard solution: 20 µg/mL of USP Desmopressin Acetate RS in Mobile phase
Sample solution: 20 µg/mL of Desmopressin Acetate in Mobile phase
System suitability solution: Dissolve about 1 mg of USP Oxytocin Identification RS. accurately weighed, in a 50-mL volumetric flask, dilute with Mobile phase to volume, and mix. Transfer 5.0 mL each of the resulting solution and the Sample solution to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix.
Chromatographic system
- (See Chromatography (621), System Suitability.)
- Mode: LC
- Detector: UV 220 nm
- Column: 4.6-mm x 25-cm; 5-µm packing 11
- Column temperature: 30o
- Flow rate: 1.0 mL/min
- Injection volume: 50 µL
System suitability
- Samples: Standard solution and System suitability solution
- Suitability requirements
- Resolution: NLT 1.5 between desmopressin and oxytocin, System suitability solution
- Tailing factor: NMT 2.0, Standard solution
- Relative standard deviation: NMT 2.0% for the desmopressin peak area for replicate injections, Standard solution
- Chromatogram similarity: The desmopressin peak elutes before the oxytocin peak, System suitability solution.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of desmopressin (C46H64N14O12S2) in the portion of Desmopressin Acetate taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Desmopressin Acetate RS (calculated on the anhydrous, acetic acid-free basis) in the Standard solution (mg/ml.)
Cu = concentration of Desmopressin Acetate (calculated on the anhydrous, acetic acid-free basis) in the Sample solution (mg/mL)
Acceptance criteria: 95.0%-105.0% on the anhydrous, acetic acid-free basis
4 IMPURITIES
4.1 DESMOPRESSIN-RELATED IMPURITIES
Mobile phase and System suitability solution: Prepare as directed in the Assay.
Standard solution: 1 µg/mL of USP Desmopressin Acetate RS in Mobile phase, prepared by diluting 0.5 mL of the Standard solution from the Assay with Mobile phase to 10 mL
Sample solution: 200 µg/mL of Desmopressin Acetate in Mobile phase
Chromatographic system
- (See Chromatography (621), System Suitability.)
- Mode: LC
- Detector: UV 220 nm
- Column: 4.6-mm x 25-cm; 5-µm packing 11
- Column temperature: 30°
- Flow rate: 1.0 mL/min
- Injection volume: 200 µL
System suitability
- Samples: System suitability solution and Standard solution
- Suitability requirements
- Resolution: NLT 1.5 between desmopressin and oxytocin, System suitability solution
- Tailing factor: NMT 2.0, Standard solution
- Relative standard deviation: NMT 5.0% for the desmopressin peak area for replicate injections, Standard solution
- Chromatogram similarity: The desmopressin peak elutes before the oxytocin peak, System suitability solution.
Analysis
Samples: Standard solution and Sample solution
Record the chromatograms, and measure the response for each peak, except for the main desmopressin peak of the Sample solution.
Calculate the percentage of each individual impurity in the portion of Desmopressin Acetate taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of each individual impurity from the Sample solution
rs = peak response of desmopressin from the Standard solution
Cs = concentration of USP Desmopressin Acetate RS (calculated on the anhydrous, acetic acid-free basis) in the Standard solution (mg/mL)
Cu = concentration of Desmopressin Acetate (calculated on the anhydrous, acetic acid-free basis) in the Sample solution (mg/mL)
Acceptance criteria
- Any individual impurity: NMT 0.5%
- Total impurities: NMT 1.5%
5 OTHER COMPONENTS
ACETIC ACID IN PEPTIDES (503): 3.0%-8.0%
6 SPECIFIC TESTS
MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIC MICROORGANISMS (62): The total aerobic microbial count does not exceed 102 cfu/g.
WATER DETERMINATION (921), Method I, Method Ic: NMT 6.0%
BACTERIAL ENDOTOXINS TEST (85): The level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Desmopressin Acetate is used can be met. Where the label states Desmopressin Acetate must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement under the relevant dosage form monograph(s) in which Desmopressin Acetate is used can be met.
7 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, preferably of Type I glass, protected from light and moisture. Store at a temperature not exceeding 25°, preferably between 2° and 8°.
Change to read
USP Reference Standards 〈11〉
USP Desmopressin Acetate RS
USP Oxytocin Identification RS


