Deoxycholic Acid
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
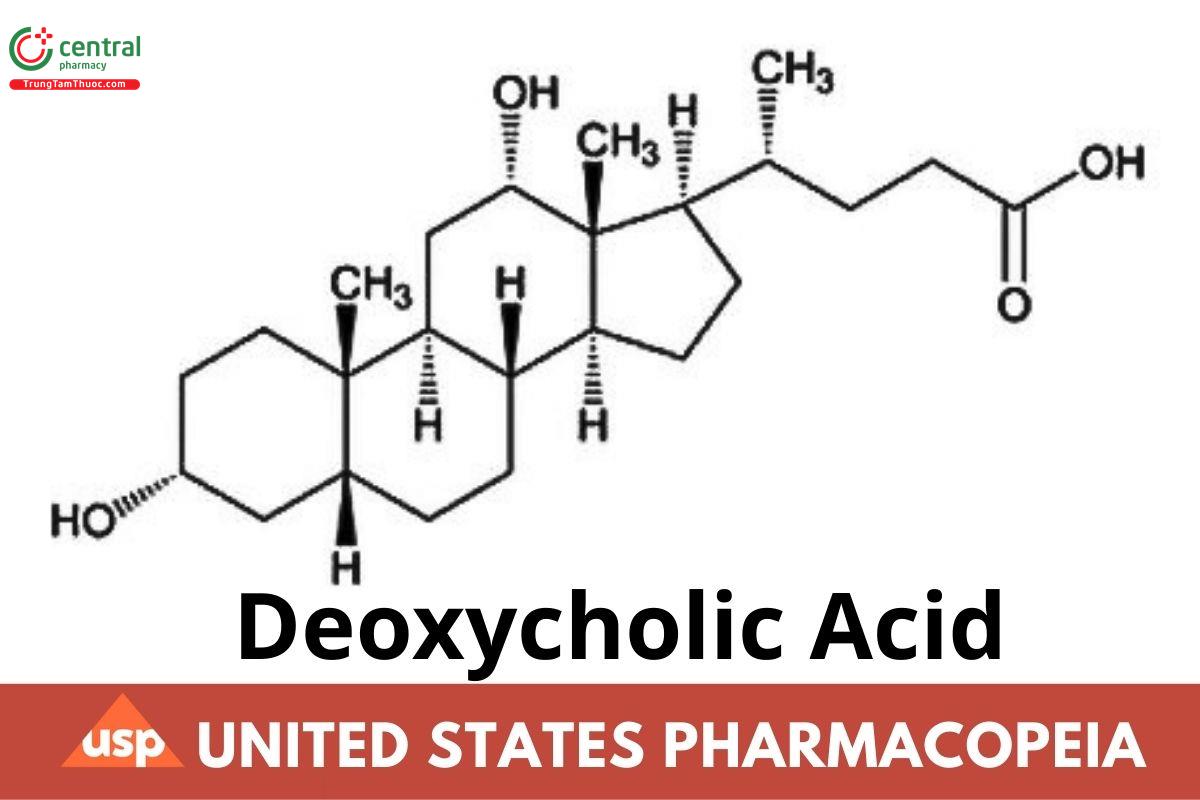
C24H40O4 392.58
Cholan-24-oic acid, 3,12-dihydroxy-, (3α,5β,12α)-;
3α,12α-Dihydroxy-5β-cholan-24-oic acid CAS RN®: 83-44-3.
1 DEFINITION
Deoxycholic Acid contains NLT 97.0% and NMT 103.0% of deoxycholic acid (C24H40O4), calculated on the anhydrous basis. Deoxycholic Acid can be of animal or synthetic origin. FDA has not approved the use of animal-derived Deoxycholic Acid as a drug substance.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K
B. The retention time of the deoxycholic acid peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Diluent: Methanol and water (80:20)
Solution A: 0.1% formic acid in water
Solution B: 0.1% formic acid in acetonitrile
Mobile phase: See Table 1.
| Table 1 | ||
| Time (min) | Solution A (%) | Solution B (%) |
| 0.0 | 75.0 | 25.0 |
| 2.0 | 55.0 | 45.0 |
| 14.0 | 42.0 | 58.0 |
| 24.0 | 0.0 | 100.0 |
| 35.0 | 0.0 | 100.0 |
Standard solution: 0.01 mg/mL of USP Deoxycholic Acid RS in Diluent
Sample solution: 0.01 mg/mL of Deoxycholic Acid in Diluent
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: Charged aerosol
- Column: 4.6-mm × 15-cm; 3-µm packing L1
- Flow rate: 1.0 mL/min
- Injection volume: 25 µL
- Run time: 35 min
System suitability
- Sample: Standard solution
- [Note-The retention time of deoxycholic acid is about 13.0 min.]
- Suitability requirements
- Relative standard deviation: NMT 3.0% for six injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of deoxycholic acid (C24H40O4) in the portion of Deoxycholic Acid taken:
Result = (ru/rs) × (Cs/Cu) × P
ru = peak area of deoxycholic acid from the Sample solution
rs = peak area of deoxycholic acid from the Standard solution
Cs = concentration of USP Deoxycholic Acid RS in the Standard solution
Cu = concentration of Deoxycholic Acid in the Sample solution
P = labeled purity of USP Deoxycholic Acid RS (%)
Acceptance criteria: 97.0%–103.0% on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
Sample: 1.0 g
Acceptance criteria: NMT 0.2%
4.2 Limit of Lead
[NOTE-This test and Acceptance criteria are only applicable to Deoxycholic Acid of animal origin. Select reagents having as low a lead content as practicable, and store all solutions in high-density polyethylene containers. Rinse all plastic and glassware thoroughly with warm, 50% nitric acid followed by water.]
Standard solutions: [NOTE-Prepare these solutions on the day of use.] Transfer 10.0 and 50.0 mL of standard lead solution TS into two separate 100-mL volumetric flasks, add 10 mL of 3 N hydrochloric acid to each, and dilute with water to volume. The third standard, 10.0 µg/mL, is taken directly from standard lead solution TS.
Sample solution: Transfer 10,0 g of Deoxycholic Acid, weighed to the nearest 0.1 mg, into an evaporating dish. Add 5 mL of 25% sulfuric acid (made by adding 25 mL of sulfuric acid to 75 mL of water), and distribute the 25% sulfuric acid solution uniformly. Within a hood, place the dish on a steam bath to evaporate most of the water. Place the dish on a burner, and slowly pre-ash the remaining Sample solution by expelling most of the sulfuric acid. Place the dish in a muffle furnace that has been set at 525°, and ash the contents of the dish until the residue appears free from carbon. Cool, and cautiously wash down the inside of the evaporation dish with water. Add 5 mL of 1 N hydrochloric acid. Place the dish on a steam bath, and evaporate to dryness. Add 1.0 mL of 3 N hydrochloric acid and approximately 5 mL of water, and heat briefly on a steam bath to dissolve any residue. Transfer to a 10-mL volumetric flask, dilute with water to volume, and mix.
Sample blank: Prepare by ashing 5 ml of 25% sulfuric acid solution. Cool, and cautiously wash down the inside of the evaporation dish with water. Add 5 mL of 1 N hydrochloric acid. Place the dish on a steam bath, and evaporate to dryness. Add 1.0 mL of 3 N hydrochloric acid and approximately 5 mL of water, and heat briefly on a steam bath to dissolve any residue. Transfer to a 10-mL volumetric flask, dilute with water to volume, and mix.
Instrumental conditions
- (See Atomic Absorption Spectroscopy 〈852〉.)
- Mode: Atomic absorption
- Analytical wavelength: 283.3 nm
- Lamp: Lead electrodeless discharge
- Flame: Air-acetylene
- Slit width: 0.7 nm
- Instrument blank: Water
Standard curve
- Samples: Standard solutions and Sample blank
- Plot: Corrected absorbance values versus their corresponding concentration (µg/mL). [NOTE-Determine corrected absorbance values by subtracting the absorbance of the Sample blank from the absorbance of the Standard solutions.]
Analysis
Samples: Sample solution and Sample blank
[NOTE-Determine corrected absorbance values by subtracting the absorbance of the Sample blank from the absorbance of the Sample solution.]
From the Standard curve, determine the lead concentration in the Sample solution.
Calculate the lead content in the portion of Deoxycholic Acid taken:
Result = (Cs × V)/W
Cs = concentration of lead from the Standard curve (µg/mL)
V = final volume of the sample (mL)
W = weight of the sample taken (g)
Acceptance criteria: NMT 4 µg/g
4.3 Organic Impurities
Diluent, Mobile phase, Standard solution, and Chromatographic system: Proceed as described in the procedure for Assay.
Sensitivity solution: 0.5 µg/ml. of USP Deoxycholic Acid RS in Diluent
Cholic acid solution: 0.01 mg/mL of USP Cholic Acid RS in Diluent
Sample stock solution: 1.0 mg/mL of Deoxycholic Acid in Diluent
Sample solution: 0.01 mg/mL of Deoxycholic Acid in Diluent prepared from the Sample stock solution
System suitability
- Samples: Standard solution and Sensitivity solution
- [NOTE-The retention time of deoxycholic acid is about 13.0 min. The relative retention times for cholic acid and deoxycholic acid are 0.56 and 1, respectively. The relative retention times for other impurities are listed in Table 2.1
- Suitability requirements
- Relative standard deviation: NMT 3.0% for six injections, Standard solution
- Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Cholic acid solution, Sample solution, and Sample stock solution
Calculate the percentage of each impurity in the portion of Deoxycholic Acid taken:
Result = [ ru/(rs× 100 + rT) ] × 100
ru = impurity peak area
rs = deoxycholic acid peak area (Sample solution)
rT = total impurity peak areas (Sample stock solution)
Acceptance criteria
Deoxycholic Acid of animal origin
Cholic acid: NMT 1.0%
Total impurities: NMT 2.0%
Deoxycholic Acid of synthetic origin: See Table 2.
| Table 2 | ||
| Name | Relative Retention Time | Acceptance Criteria NMT (%) |
| 3α,12β-Dihydroxy-5β-cholan-24-oic acid | 0.69 | 0.15 |
| 3α,12α-Dihydroxy-5β-chol-9(11)-en-24-oic acid | 0.87 | 0.15 |
| Ethyl 3α,12α-dihydroxy-5β-cholan-24-oate | 1.61 | 0.15 |
| Any unspecified impurity | - | 0.10 |
| Total impurities | - | 2.0 |
5 SPECIFIC TESTS
5.1 OPTICAL ROTATION (781S), Procedures, Specific Rotation
Sample solution: 1% (w/v) solution in ethanol
Acceptance criteria
- Deoxycholic Acid of animal origin: NLT +55°
- Deoxycholic Acid of synthetic origin: +50° to +60°
5.2 WATER DETERMINATION (921), Method I. Method ic: NMT 1.0%
5.3 CLARITY OF SOLUTION
[NOTE-If intended for use in preparing parenteral dosage forms, it meets the requirements of the Clarity of Solution test.]
Hydrazine solution: 10 mg/mL of hydrazine sulfate. Allow to stand 4-6 h before use.
Methenamine solution: Transfer 2.5 g of methenamine to a 100-ml. glass-stoppered flask, add 25.0 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension: To the flask containing Methenamine solution add 25.0 mL of the Hydrazine solution, mix, and allow to stand for 24 h. This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use.
Opalescence standard: Transfer 15.0 mL of the Primary opalescent suspension to a 1000-mL volumetric flask, and dilute with water to volume. Use this suspension within 24 h after preparation.
Reference suspension: Transfer 5.0 mL of the Opalescence standard to a 100-mL volumetric flask, and dilute with water to volume.
Sample solution: 5 mg/mL of Deoxycholic Acid in 0.1 N sodium hydroxide
Analysis: Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15-25 mm to obtain a depth of 40 mm. Similarly transfer a portion of the Reference suspension to a separate matching test tube. Compare the Sample solution and the Reference suspension in diffused daylight, viewing vertically against a black background (see Visual Comparison (630)) for 5 min after preparation of the Reference suspension.
Acceptance criteria: The Sample solution is not more opalescent than the Reference suspension.
5.4 COLOR OF SOLUTION
[NOTE-If intended for use in preparing parenteral dosage forms, it meets the requirements of the Color of Solution test.)
Diluent: 27.5 ml. of hydrochloric acid in 1000 mL of water
Standard stock solutions: Prepare two solutions, A and B, containing, respectively, the following parts of ferric chloride CS, cobaltous.chloride CS. cupric sulfate CS, and Diluent.
- Standard stock solution A: 2.4: 0.6: 0: 7.0
- Standard stock solution B: 2.4: 1.0: 0.4: 6.2
Standard solutions
- [NOTE-Prepare and use these solutions immediately.]
- Standard solution A: Transfer 2.5 mL of Standard stock solution A to a 100-mL volumetric flask, dilute with Diluent to volume, and mix.
- Standard solution B: Transfer 2.5 mL of Standard stock solution B to a 100-ml, volumetric flask, dilute with Diluent to volume, and mix.
Sample solution: 5 mg/mL of Deoxycholic Acid in 0.1 N sodium hydroxide
Analysis: Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15-25 mm, to obtain a depth of 40 mm. Similarly transfer portions of Standard solution A and Standard solution B to separate matching test tubes. Compare the color of the Sample solution with that of Standard solution A and Standard solution B in diffused daylight, viewing vertically against a white background (see Visual Comparison (630)).
Acceptance criteria: The Sample solution is not more intensely colored than Standard solution A and Standard solution B.
5.5 BACTERIAL ENDOTOXINS TEST (85)
If labeled for use in preparing parenteral dosage forms, it meets the requirements of NMT 240 Endotoxin Units/g.
5.6 MICROBIAL ENUMERATION TESTS (61) and TESTS FOR SPECIFIED MICROORGANISMS (62):
If labeled for use in preparing parenteral dosage forms, the total aerobic microbial count does not exceed 100 cfu/g, and the total combined molds and yeasts count does not exceed 10 cfu/g
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers. No storage requirements specified.
LABELING: Label it to indicate whether Deoxycholic Acid is derived from an animal or synthetic source. Deoxycholic Acid intended for use in preparing parenteral dosage forms is so labeled.
USP REFERENCE STANDARDS (11)
- USP Cholic Acid RS
- USP Deoxycholic Acid RS
- USP Endotoxin RS


