Deferoxamine Mesylate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
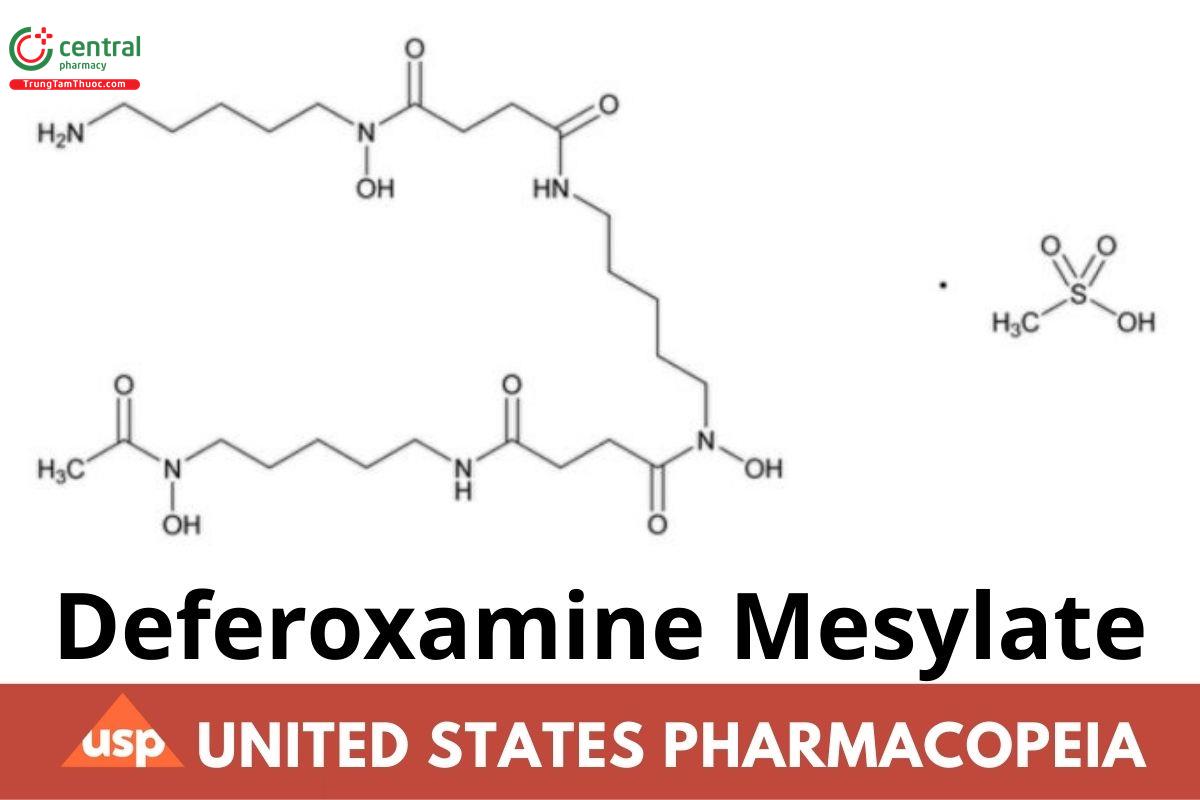
C25H48N6O8·CH4O3S 656.79
Butanediamide, N′-[5-[[4-[[5-(acetylhydroxyamino)pentyl]amino]-1,4-dioxobutyl]hydroxyamino]pentyl]-N-(5-aminopentyl)-N-hydroxy-, monomethanesulfonate;
N-[5-[3-[(5-Aminopentyl)hydroxycarbamoyl]propionamido]pentyl]-3-[[5-(N-hydroxyacetamido)pentyl]carbamoyl]propionohydroxamic acid monomethanesulfonate (salt)
CAS RN®: 138-14-7; UNII: V9TKO7EO6K.
1 DEFINITION
Deferoxamine Mesylate contains NLT 93.0% and NMT 102.0% of deferoxamine mesylate (C25H48N6O8·CH4O3S), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Solution A: 1.32 g/L of dibasic ammonium phosphate in water. Adjust with phosphoric acid to a pH of 3.0.
Solution B: Acetonitrile and Solution A (1:1)
Mobile phase: See Table 1.
| Table 1 | ||
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 88 | 12 |
| 20 | 80 | 20 |
| 35 | 57.5 | 42.5 |
| 35.1 | 88 | 12 |
| 40 | 88 | 12 |
Diluent: Acetonitrile and water (6:94)
Standard solution: 1.0 mg/mL of USP Deferoxamine Mesylate RS in Diluent
Sample solution: 1.0 mg/mL of Deferoxamine Mesylate in Diluent
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 220 nm
- Column: 4.6-mm × 7.5-cm; 3.5-µm packing L1
- Temperatures
- Column: 32°
- Autosampler: 5°
- Flow rate: 1.5 mL/min
- Injection volume: 20 µL
- System suitability
- Sample: Standard solution
- Suitability requirements
- Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of deferoxamine mesylate (C25H48N6O8·CH4O3S) in the portion taken:
Result = (ru/rs) × (Cs/Cu) × 100
ru = peak response of deferoxamine from the Sample solution
rs = peak response of deferoxamine from the Standard solution
Cs = concentration of the Standard solution (mg/mL)
Cu = concentration of the Sample solution (mg/mL)
Acceptance criteria: 93.0%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
NMT 0.1%, 2.0 g being used
4.2 Chloride and Sulfate, Chloride 〈221〉
NMT 0.012%; a 1.2-g portion shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid.
4.3 Chloride and Sulfate, Sulfate 〈221〉
NMT 0.04%; a 0.5-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid.
4.4 Organic Impurities
Solution A, Solution B, Diluent, Mobile phase, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard stock solution: Use the Standard solution, prepared as directed in the Assay. [Note-USP Deferoxamine Mesylate RS contains impurity A as a minor component.]
Standard solution: 0.01 mg/mL of USP Deferoxamine Mesylate RS in Diluent from the Standard stock solution
System suitability
- Samples: Standard stock solution and Standard solution
- Suitability requirements
- Resolution: NLT 2.0 between the impurity A and deferoxamine peaks, Standard stock solution
- Relative standard deviation: NMT 5.0% for the deferoxamine peak, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Deferoxamine Mesylate taken:
Result = (ru/rs) × (Cs/ Cu) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of deferoxamine from the Standard solution
Cs = concentration of the Standard solution (mg/mL)
Cu = concentration of the Sample solution (mg/mL)
Acceptance criteria: see Table 2
[Note-The reporting level for impurities is 0.04%.]
| Table 2 | ||
| Name | Relative Retention Time | Acceptance Criteria NMT (%) |
| Impurity Aa,b | 0.85-0.87 | 3.0c |
| Deferoxamine | 1.0 | - |
| Any unspecified impurity | - | 1.0 |
| Total impurities eluting | - | 5.0 |
| after deferoxamine | - | 2.0 |
a Des-methylene impurity (Desferrioxamine A1 and/or other desferrioxamines).
b All des-methylene impurities that elute in the 0.85–0.87 range should be treated as a single impurity. Where the cluster of unresolved peaks in this range is present, it should be integrated together as one peak to determine compliance.
c The acceptance criterion of NMT 3.0% applies to the sum of the peaks in the specied range.
5 SPECIFIC TESTS
pH 〈791〉: 4.0–6.0, in a solution (1 in 100)
Water Determination, Method I 〈921〉: NMT 2.0%
Sterility Tests 〈71〉: Meets requirements if labeled sterile
Bacterial Endotoxins Test 〈85〉: NMT 0.33 USP EU/mg if sterile or for injectable preparation
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at room temperature.
Labeling: If intended for injectable dosage form preparation, label indicates sterile or requires further processing.
USP Reference Standards 〈11〉
USP Deferoxamine Mesylate RS


